Transition Metals
advertisement

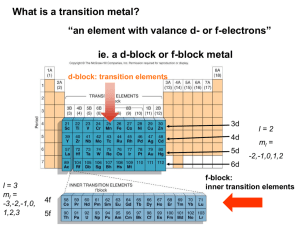
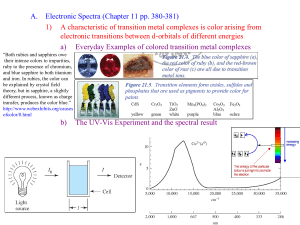
CH# 17 Coordination Chemistry Transition Metals Transition metals show similarities within a period and a group, different than representative elements Differences can be attributed to the fact that when electrons are added across a period the valence electrons are not effected. Therefore group designations are not important here Behave as metals, strong metallic character Transition Metals • Some differences – Melting point, Tungsten melts 3400°, while mercury 39°C – Some soft, like sodium that can be cut with a butter knife – Reactivity • Some spontaneously react with oxygen like iron, which flakes off • Others react with oxygen to make a colorless tight fitting oxide, such as chromium, thus protecting the surface • Some metals are inert to oxygen such as gold, silver and platinum Transition Metals Ionic compound formation More than one oxidation state is often observed Cations, often are complexes, which we will discuss later in this chapter Most compounds are colored, since complexes absorb visible light Many compounds are paramagnetic This chapter will deal specifically the first row transition elements Transition Elements Electron Configurations Exceptions to the AUFBAU principle Cr prefers a half full d as opposed to a full 4s, thus 4s13d5 Copper prefers a full 3d as opposed to a full 4s, thus 4s13d10 This half filled, or filled d orbital, is used most of the time to explain this, but other transition metals do not follow this trend. Electron Configurations Many texts explain AUFBAU exceptions of chromium and copper as a half full sublevel are more stable than a full 4 s sublevel, or for copper that a full dsublevel is more stable than a half full 4s Why is this not the case in periods below? The 4 s and the 3 d orbitals are of about the same energy or nearly degenerate. Perhaps there is a larger repulsive force in the 4s than in 3d orbitals. I do not think any one knows, but it is good to think and create right? Electron Configurations 4d and 5d Transition Series – See the size relation on next slide • Decrease in size as we go from left to right, stopping when the d is half full • Significant drop in size going from 3d to 4d, but 4d and 5d remain about the same size –Called Lanthanide contraction –Adding f electrons below the d and the valence shell shel electrons (shielding) –Thus the effect of the increasing size by adding another shell of electrons, which is normally in transition and representative elements, is offset by the shielding of the added f electrons Transition Element Sizes Oxidation States and IE See common oxidation states on Next slide The maximum oxidation state for each transition element going across the row is what we would get by losing both 4s and 3d electrons, toward the end only 2+ is observed, the explanation is that as the effective charge increases thus holding the d electrons tighter. Reducing ability, decreases from left to right Transition Metal Oxidations #’s Sc V 3 2 3 4 5 Ti Cr Mn Fe Co Ni Cu Zn 2 3 4 4 2 3 5 6 2 3 4 2 3 6 7 6 2 3 2 3 1 2 3 2 Ionization Energies Red dot- First ionization energy (removing 4s e) Blue dot-third ionization energy removing 3d electron, closer to nucleus, thus more tightly held First-row Transition Metals Scandium Rare element most always +3 oxidation state, ie ScCl3, Sc2O3 Chemistry of scandium resembles the lanthanides Colorless compounds Diamagnetic Color and magnetic properties are due to d electron, Sc has no d electrons First-row Transition Metals Titanium Found in the earths crust (0.6%) Low density and high strength Fairly inert, and is used in pipes TiO2 is a very common white pigment Common oxidation state is +4 First-row Transition Metals Vanadium Found in the earth’s crust about 0.02% Common oxidation state is +5 Since vanadium contains d electrons solutions are colored VO2+ is yellow with V in the +5 oxidation state VO2+ is blue with V in the +4 oxidation state V3+ is blue-green with V in +3 oxidation state V2+ is violet with V in +2 oxidation state First-row Transition Metals Chromium – Rare, but important industrial chemical – Chromium oxide is colorless, tuff, and holds to the metal strongly, almost invisible – Chromium compounds in solution are also colored since they contain d electrons – Common oxidation states are +2, +3 and +6 – Chromium VI is an excellent oxidizing agent! Why? • Strength increases as acidity increases • Chromerge very good glassware cleaning agent – What would we predict for Cr metal? – Cr6+ in the form of dichromate ion usually reduces to the +3 state First-row Transition Metals • Iron – Is the most abundant heavy metal (4.7%) in earth’s crust, Why? – Common oxidation states +2 and +3 – Iron solutions are colored since they contain d electrons • Cobalt – Relatively rare – Hard bluish-white metal – Common oxidation states are +2 and +3 – Oxidation states +1 and +4 are also known • Typical color is rose color First-row Transition Metals Nickel Most always the +2 oxidation state Sometimes +3 oxidation state Emerald green colored solutions First-row Transition Metals • Copper – Quite common, as sulfides, arsenides, chlorides and carbonates – Great electrical conductor second only to silver – Widely used in plumbing – Found in bronze and brass – Not highly reactive will not reduce H+ – Slowly oxides in air, producing a green oxide – Common oxidation state +2, +1 is also known – Aqueous solution are bright Royal blue – Quite toxic, used to kill bacteria – Paint often contains copper so algae do not grow on the paint First-row Transition Metals Zinc Quite common in earths crust, usually as ZnS Great reducing agent, quite reactive Oxidation state of +2 Used to galvanize steel Coordination compounds Transition metals form coordination compounds Transition metals contain a complex ion attached to ligands via coordinate covalent bonds Coordination compounds are usually colored and paramagnetic Coordination compounds Complex ions, usually inside [ ] Transition coordinately bonded to Lewis bases, the metal is acting as a Lewis acid Example [CoCl(NH3)5]2+ this cation can combine with anions to balance the charge, thus forming a salt Ligands are the groups of atoms bonded with a coordinate covalent bond to a transition metal, or a transition metal ion. Coordinate Covalent Bonding Coordinate Covalent Bonding Coordinate Covalent Bonding Coordinate Covalent Bonding Coordination compounds Alfred Werner was the father of coordination chemistry Alfred Werner called the salt formation the primary valence The secondary valence is the formation of the complex ion itself The compound above has a secondary valence of 6, since it combines with 6 ligands The primary valence is +2 since that is what needs to be neutralized with anions. Now days the secondary valence is called the coordination number and the primary valence is called the oxidation state Aqueous Solutions of Metal Ions Coordination Compounds The number of coordinate covalent bonds formed by the metal ion and the ligands Variance of 2-8, with 6 being most common. Geometrical Shape Ligands = 2, then linear Rare for most metals Common for d-10 systems (Cu+, Ag+, Au+, Hg2+) Ligands = 3, Trigonal planar Rare for most metals Is known for d-10 systems (example HgI3-) Coordination Compounds • Geometrical Shape Ligands = 4, then tetrahedral, or square planar Tetrahedral structure is observed for nontransition metals, BeF42- and d-10 inons such as ZnCl42-, FeCl4-, FeCl42 Square planar is found with second and third row transition metals with d-8 Rh+, Pd2+ -Ligands = 5 trigonal bipyramid square pyramidal Coordination compounds • Geometrical Shape − Ligands = 6, then octahedral and prismatic (rare) − Ligands = 7 Relatively uncommon, pentagonal Second and third row transition metals, lanthanides , and actinides − Lignads = 8, relatively common for larger metal ions, common geometry antiprism and dodecahedron − Lignads = 9 larger metal ions, geometry tricapped trigonal prism [Nd(H2O)9]3+ The Ligand Arrangements for Coordination Numbers 2, 4, and 6 Ligands Atoms attached to a transition metal via coordinate covalent bonds They are Lewis bases, since they donate a pair of electrons to the transition metal. Ligands are classified relative to how many attachments to the metal Monodentate forms one bond to a transition metal Lignads forming more than bond are called chelating ligands, or chelates Ligands Ligands are classified relative to how many attachments to the metal Bidentate, a chelating agent, forms two bonds, examples: Oxalate Ethylenediamine Polydentate forms more than two bonds. Diethylenetriamine Ethylenediaminetetraacetic acid Ligands EDTA is used to remove lead from animals More complicated ligands are found in biological compounds EDTA is used as a preservative to tie up substances that could catalyze decomposition of food products Ethylenediamine Ethylenediamminetetracidic acid Coordination of EDTA with + a 2 Metal Ion Nomenclature Cationic species named before anionic species Within a complex, the ligands are named first in alphabetical order followed by the metal atom the names of anionic lignads end in the suffix -o chloride ----->chloro cyanide ----->cyano oxide ----->oxo Hydroxide -->hydroxo Oxalate------>oxalato Sulfate ------>Sulfato Nitrate ------>Nitrato Nomenclature lignads whose names end in -ite or ate become -ito and ato respectively carbonate ----> carbonato oxalate-----> oxalato thiosulfate ----> thiosulfato Sulfite -----> sulfito neutral lignads are given the same names as the neutral molecule exceptions, ammonia (ammine), water (aqua), carbon monoxide (Carbonyl), and NO (nitrosyl) Nomenclature When there is more than one of a particular ligand, number is specified by di, tri, tetra, penta, hexa, and so forth. when confusion might result, the prefixes bis, tris and tetrakis are employed e.g. bis(ethylenediaminne) negative (anionic) complex ions always end in the suffix -ate aluminum -----> aluminate chromium -----> chromate manganese ------> manganate coblat ------> cobaltate For some metals the -ate is appended to the Latin stem always appears with Nomenclature the common English name for the element iron ----> ferr ------> ferrate copper ---> cupra -----> cuprate lead ----> plumb -----> plumbate silver ---> argent ----> argentate gold ---> aur ----> aurate tin ----> stann -----> stannate the oxidation number of the metal in the complex is written in roman numerals within parentheses following the name of the metal Nomenclature Formula writing Metal is first, followed by anions, then neutral molecules If two or more anions or neutral molecules are present, then use alphabetical order. Nomenclature Examples tetracyanonickelate(II) ion tetramminedichlorocobalt(III) ion sodium hexanitratochromate(III) diamminesilver(I) ion Nomenclature Formula writing Metal is first, followed by anions, then neutral molecules If two or more anions or neutral molecules are present, then use alphabetical order. Nomenclature Examples tetracyanonickelate(II) ion tetramminedichlorocobalt(III) ion sodium hexanitratochromate(III) diamminesilver(I) ion [Ni(CN)4]2- Nomenclature Formula writing Metal is first, followed by anions, then neutral molecules If two or more anions or neutral molecules are present, then use alphabetical order. Nomenclature Examples tetracyanonickelate(II) ion tetramminedichlorocobalt(III) ion sodium hexanitratochromate(III) diamminesilver(I) ion [Ni(CN)4]2[CoCl2(NH3)4]+ Nomenclature Formula writing Metal is first, followed by anions, then neutral molecules If two or more anions or neutral molecules are present, then use alphabetical order. Nomenclature Examples tetracyanonickelate(II) ion tetramminedichlorocobalt(III) ion sodium hexanitratochromate(III) diamminesilver(I) ion [Ni(CN)4]2[Co(NH3)4Cl2]+ Na3[Cr(NO3)6] Nomenclature Formula writing Metal is first, followed by anions, then neutral molecules If two or more anions or neutral molecules are present, then use alphabetical order. Nomenclature Examples tetracyanonickelate(II) ion [Ni(CN)4]2 tetramminedichlorocobalt(III) ion [CoCl2 NH3)4]+ sodium hexanitratochromate(III) Na3[Cr(NO3)6] diamminesilver(I) ion [Ag(NH3)2]+ Nomenclature Formula writing Metal is first, followed by anions, then neutral molecules If two or more anions or neutral molecules are present, then use alphabetical order. Nomenclature Examples tetracyanonickelate(II) ion [Ni(CN)4]2 tetramminedichlorocobalt(III) ion [CoCl2(NH3)4]+ sodium hexanitratochromate(III) Na3[Cr(NO3)6] diamminesilver(I) ion [Ag(NH3)2]+ Nomenclature Name the following: [Ni(H2O)6]Cl2 hexaaquanickel(II) chloride [Cr(en)3](ClO3)3 K4[Mn(CN)6] K[PtCl5 (NH3)] [Cu(en)(NH3)2][Co(en)Cl4] [Pt(en)2Br2](ClO4)2 Nomenclature Name the following: [Ni(H2O)6]Cl2 hexaaquanickel(II) chloride [Cr(en)3](ClO3)3 tris(ethylenediamene)chromium(III) chlorate K4[Mn(CN)6] K[PtCl5(NH3)] [Cu(en)(NH3)2][Co(en)Cl4] [Pt(en)2Br2](ClO4)2 Nomenclature Name the following: [Ni(H2O)6]Cl2 hexaaquanickel(II) chloride [Cr(en)3](ClO3)3 trisethylenediamenechromium(III) chlorate K4[Mn(CN)6] Potassium hexacyanomanganate(IV) K[PtCl5(NH3)] [Cu(en)(NH3)2][Co(en)Cl4] [Pt(en)2Br2](ClO4)2 Nomenclature Name the following: [Ni(H2O)6]Cl2 hexaaquanickel(II) chloride [Cr(en)3](ClO3)3 trisethylenediamenechromium(III) chlorate K4[Mn(CN)6] Potassium hexacyanomanganate(IV) K[PtCl5(NH3)] Potassium monoaminepentachloroplatinate(IV) [Cu(en)(NH3)2][Co(en)Cl4] [PtBr2(en)2](ClO4)2 Nomenclature Name the following: [Ni(H2O)6]Cl2 hexaaquanickel(II) chloride [Cr(en)3](ClO3)3 trisethylenediamenechromium(III) chlorate K4[Mn(CN)6] Potassium hexacyanomanganate(IV) K[PtCl5(NH3)] Potassium triaminpentachloroplatinate(IV) [Cu(en)(NH3)2][Co(en)Cl4] ethylenediaminediaminecopper(II) tetrachloroethylenediaminecobaltate(II) [Pt(Br2en)2](ClO4)2 Nomenclature Name the following: [Ni(H2O)6]Cl2 hexaaquanickel(II) chloride [Cr(en)3](ClO3)3 tris(ethylenediamene)chromium(III) chlorate K4[Mn(CN)6] Potassium hexacyanomanganate(IV) K[PtCl5 (NH3)] Potassium monoaminpentachloroplatinate(IV) [Cu(en)(NH3)2][Co(en)Cl4] ethylenediaminediamincopper(II) tetrachloroethylenediaminecobaltate(II) [Pt(en)2Br2](ClO4)2 bis(ethylenediamine)dibromoplatinum(IV) perchlorate Some Classes of Isomers Coordination Isomers Structural (constitutional) isomers Definition- Different compounds of the same formula. Types of structural (constitutional) isomers Ionization isomerism [Cr(NH3)SO4]Cl ppts AgCl when silver nitrate is added [Cr(NH3Cl)]SO4 ppts barium sulfate when barium is added Hydrate isomers differ in the placement of water Coordination isomers differ in the placement of ligands between the metal atoms [CuBr4][PtCl4] or [CuCl4][PtBr4] Coordination Isomers Types of structural (constitutional) isomers Linkage isomers differ by the atom that is coordinated to the metal O-N-O-M O2N-M, bond to oxygen, or bond to nitrogen Stereoisomerism (octahedral use models) Definition, Same formula, same attachment of atoms, but atoms are in different volumes of space geometrical isomers (cis and trans) cis-[Co(NH3)4Cl2]+ chlorides on same side trans- chlorides on the opposite side As a Ligand, NO2- can Bond to a Metal Ion (a) Through a Lone Pair on the Nitrogen Atom or (b) Through a Lone Pair on One of the Oxygen Atoms (a) The cis Isomer of Pt(NH3)2Cl2 (b) The trans Isomer of Pt(NH3)2Cl2 The Compound [Co(NH3)4Cl2]Cl has cis and trans Isomers Optical Isomers Optical isomers(nonsuperimposable mirror images) Chiral molecule-nonsuperimposable on it’s mirror image Enantiomers-a pair of nonsuperimposable mirror images Ploarimeter-an instrument that measures the rotation of polarized light by an optically active compound Dextrorotatory-rotation of polarized light clockwise Levorotatory-rotation of polarized light counterclockwise Racemates- a 50/50 mixture of enantiomers (no rotation of polarized light) Optical Isomers A human hand exhibits a nonsuperimposable mirror image. Note that the mirror image of the right hand (while identical to the left hand) cannot be turned in any way to make it identical to (superimposable on) the actual right hand. Optical Isomers Isomers I and II of CO(en)33+ are Mirror Images that Cannot be Superimposed Optical Isomers (a) Superimposable. (b) Not Superimposable Polarized Light Polarized Light Rotating the Plane of Polarization of Light Valence Bond Model Valence bond (VB) approach (Localized Model) relative to octahedral systems Overlay of the atomic orbitals of the metal and the ligands Since the ligands normally do not possess single electrons, then a pair of electrons from the ligand, must overlap with the empty orbitals of the metal Valence Bond Model 3d 4s 4p Consider for example the blue-violet [Cr(H2O)6]3+ complex This is a 3d3 system, thus the 6 electron pairs from the water will occupy the d2sp3 hybrid (note the 4s and 4p orbitals are used here) The 4d orbitals are not used in this case, since the 3d orbitals are lower in energy, and the bonds formed are stronger The orbital notation shows three single electrons, verified by Gouy Balance measurements. Gouy Balance Valence Bond Model Consider next the [Ni(H2O)6]2+ 4s This is a d8 system, there are no empty 3d orbitals The 6 ligands, then will occupy the 4th energy shell sp3d2 3d 4p 4d The orbital notation shows two unpaired electrons, verified by experiment Valence Bond Model When 3d orbitals are employed, the system is referred to as an inner orbital complex; where if the 4d orbitals are used, then the system is referred to as an outer orbital complex. In the two previous examples there was no choice where the electrons are placed. Coordination Compound Bonding Co3+ is an ion that can show either inner orbital, or an outer orbital complexes Co3+ is d 6 system Pairing up the 6 electrons, will produce an inner orbital d2sp3, which is diamagnetic If the d electrons are not paired, then an outer orbital sp3d2 complex is formed, with 4 unpaired electrons and paramagnetic Valence Bond Model To pair, or not to pair, that is the question This question arises for d 4,5,6 systems Failure to pair produces outer orbital systems Two factors to consider Stronger bonds are formed from 3d orbitals, than 4d orbitals Pairing means putting two electrons in the same orbital. A higher energy system for sure (electron repulsion), but the outer orbital system does not require pairing electrons Valence Bond Model If the formation of bonds releases more energy than the pairing energy, then the inner orbital complex is preferred; if not, then the outer orbital complex is favored Most first row transition elements when combined with ligands tend to favor inner orbital complexes for d4 or d6 systems, with the exception of the ligands H2O and F-, which usually prefer outer orbital complexes Valence Bond Model The d-5 system produced the half filled system (chromium), which is weird. It is hard to disturb this stability, thus paring usually does not happen, thus these systems prefer outer orbital complexes with most complexes, but CN- is an exception Valence Bond Model Valence bond approach and other geometries Consider [Ni(CN)4]2- which is square planar and diamagnetic Here Ni2+ is a d-8 system Here pairing will create an empty d orbital, thus allowing the dsp2 hybrid orbital system to form Cyanide, is a strong ligand, as it was in the octahedral system The [CoCl4]2- complex forms the tetrahedral geometry This is a d-7 system Tetrahedral here and sp3 Valence Bond Model One of the most striking physical properties of the coordination compounds is their color, and the valence bond theory does little to explain color. Crystal Field Splitting Theory Crystal field splitting theory (CF) Developed by physics to explain impurities in crystal lattices Electrostatic bonds no coordinate covalent bonds Ligands are anions or polar particles Crystal Field Splitting Theory Another modified version of molecular orbital theory Organizes the d orbitals in order of increasing energy Organization of energy depends on the geometry of the complex ion Consider the geometry of the d orbitals page 967 As a ligand approaches in an octahedral complex, the nonbonding electrons will repel electrons found in the dz2 and dx2-y2, thus splitting the potential energy of the 5 d orbitals, this is where the name Crystal Field Splitting theory comes from Crystal Field Splitting Theory Crystal Field Splitting Theory The average energy of the d orbitals is not altered The energy difference is called Δo where the o means octahedral The two d orbitals of higher energy are called eg while the three lower energy orbitals are called t2g The eg increases in energy by 0.6, and the t2g decreases in energy by 0.4, thus total energy change is zero Crystal Field Splitting CF Energy Diagram Octahedral and Tetrahedral Splits Some d Systems Some d Systems Some d Systems Some d Systems Crystal Field Splitting The energy difference is called Δo where the o means octahedral Referred to as the crystal field splitting Absorption of light corresponds to delta, the greater the difference the more blue in color light is absorbed, all other colors are reflected The energy absorbed is related to the wave length Crystal Field Splitting Placement of electrons into these 5 psudo-molecular orbitals depends on the magnitude of Δo and the pairing energy P If Δo < P, then the next electron goes into the eg orbital, creating a high spin case If Δo > P, then the next electron goes into t2g creating low spin case Examples With Pairing Energy Crystal Field Splitting Factors affecting magnitude of Δo Charge on metal ion Increasing charge causes radius to decrease, thus ligands are more strongly attached, thus increasing Δo The Δo for a tripositive ion is about 50% when compared to a dipositive ion Principle quantum number With the same charge and same ligands, then as we travel down a group then Δo increases This effect is due to the larger radius of the metal ion Repulsive ligand forces are important in smaller metal ions Crystal Field Splitting Nature of the ligands For ligands of the same group, the Δo decreases as the size of the ligand increases Smaller more localized charges interact more strongly with the d orbitals of the metal ion. Small neutral ligands with a localized pair of electrons, i.e. NH3, gives larger than expected Δo, when compared to a spherical ligand such as F-, that has unlocalized electrons Crystal Field Splitting If the ligands cause a large splitting (large value of Δ) then the electrons will fill the lower t2g orbital first, thus minimizing the single electrons (strong field case) (low spin) If the ligands cause a small splitting (low value of Δ) then the electrons will fill the t2g one at a time and then fill the eg orbitals one at a time (Weak field case) (high spin) Low Spin d-4 System High Spin d-4 System Crystal Field Splitting Only d 4,5,6,7 have a choice of high spin, or low spin This model explains magnetic and color properties of complexes. Color chart or color wheel Color Wheel Visible Spectrum Crystal Field Splitting Magnitude of splitting Magnitude of ΔO depends on the charge of the central metal, the higher the greater ΔO For example NH3 is weak field with Co 2+ and strong field with Co 3+ As charge increases the ligands are drawn closer to the metal, thus the closer the greater the splitting Crystal Field Splitting Depends on polarity, size, etc. I-<Br-<Cl-<acetate<F-<OH-<oxalate<H2O<SCN<NH3<en<NO2-<CN-≈CO Iodine is the smallest splitting Crystal Field Splitting Cobalt here can have two possibilities Depends what the ligands are High spin same as outer Low spin same as inner If iodine is used we have high spin Because delta is not large Crystal Field Splitting Bond formation overcomes small delta If aqua is used we have the low spin case All paired Diamagnetic Different colors because delta is different The possibility of high spin or low spin exists when D=4,5,6 or 7, Choice between High and Low spin d 0,1,2,3,8,9,10 High spin only Crystal Field Splitting Crystal Field Stabilization Energies If the t2g populated, then stability is increased, since it is lower potential energy Stabilization can be calculated by multiplying the number of electrons in the t2g orbital by 0.4Δ If a combination occupied by t2g and eg then subtract 0.4 t2g from 0.6 eg for stabilization energy Wave numbers Energies obtained by spectroscopic measurements are oftern given in units of wave numbers (cm-1) Wave number is the reciprocal of the wavelength of the corresponding electromagnetic radiation expressed in cm cm-1 = 11.96 j Crystal Field Splitting Color and the Colors of Complexes Two types of mixing colors Additive, occurs when colored lights are superimposed on each other Subtractive, occurs when colored paints are mixed with each other Crystal Field Splitting Additive Mixing (light beams) For additive mixing a primary color is defined as any three colors that produce white light Examples: R+G+B=W Secondary colors are those that are produced by combining two primary colors Examples: R+G=Y R+B=M Crystal Field Splitting Additive and Subtractive Mixing Complex Solutions Subtractive Mixing Some wave lengths of white light are removed from absorption (promoting electrons to higher levels) The reflected light, does not contained the absorbed colors, thus has a color due to the absence of another color. Here primary colors are M,Y, and BG; while secondary colors are G, B, and R. If a material absorbs all three primary colors, then there in no light left to be reflected, thus black. Subtractive Mixing If a material absorbs one color, primary or secondary, the reflected or transmitted color is the complimentary color. Thus a magenta shirt has that color because the dye it contains strongly absorbs green light and reflects the magenta, the compliment of green (see color wheel) Color Wheel Colored Solutions Colored solutions absorb photons of white light to promote electrons to higher energy levels. White light, minus the absorbed color, is no longer white, but appears as the compliment of the color that was absorbed Ions having noble gas configurations do not have energy absorptions in the visible range, thus they appear Colored Solutions Crystal field splitting deals with d-electrons and not Noble gas structures. The do absorb in the visible spectrum and we see the color of the light that is complimentary to the color absorbed A solution containing [Cu(H2O)4]2+ absorbs most strongly in the yellow region of the spectrum (about 580 nm) The wavelength of the transmitted light is violet Solubility of Ionic Compounds When a precipitate forms the solution is said to be saturated Saturated solutions can also be formed by adding to much solute An equilibrium exists between ions forming solid and the solid dissolving to form ions. From the equilibrium constant we can determine the molar solubility Silver Chloride Solubility Silver chloride is known to be insoluble according to our solubility rules, but some does dissolve. AgCl(s) Ag+(aq) + Cl-(aq) Ksp= 1.8X10 -10 Silver Chloride Solubility Silver chloride is known to be insoluble according to our solubility rules, but some does dissolve. AgCl(s) Ksp = Ag+(aq) + Cl-(aq) [Products] [Reactants] = ? Ksp= 1.8X10 -10 Silver Chloride Solubility Silver chloride is known to be insoluble according to our solubility rules, but some does dissolve. AgCl(s) Ag+(aq) + Cl-(aq) Ksp = [Ag+][Cl-] Ksp= 1.8X10 -10 Silver Chloride Solubility Silver chloride is known to be insoluble according to our solubility rules, but some does dissolve. AgCl(s) Ag+(aq) + Cl-(aq) Ksp = [Ag+][Cl-] = [X][X] Ksp= 1.8X10 -10 X2 = 1.8 X 10-10 X = 1.34 X 10-5 M Silver Chloride Solubility Silver chloride is known to be insoluble according to our solubility rules, but some does dissolve. AgCl(s) Ag+(aq) + Cl-(aq) Ksp = [Ag+][Cl-] = [X][X] Ksp= 1.8X10 -10 X2 = 1.8 X 10-10 X = 1.34 X 10-5 M 1.34 X 10-5 mole Ag+ mole AgCl 142 g AgCl mole Ag+ mole AgCl L 1.91 X 10-3 g AgCl will dissolve in a liter of water Sample Problem Determine the molar solubility of magnesium hydroxide. Sample Problem Determine the molar solubility of magnesium hydroxide. Mg(OH)2 (s) Mg2+ + 2 OH- ksp= 1.8 X 10-11 Sample Problem Determine the molar solubility of magnesium hydroxide. Mg(OH)2 (s) Ksp = [Mg2+][OH-]2 Mg2+ + 2 OHx 2x ksp= 1.8 X 10-11 Sample Problem Determine the molar solubility of magnesium hydroxide. Mg(OH)2 (s) Mg2+ + 2 OHx 2x ksp= 1.8 X 10-11 Ksp = [Mg2+][OH-]2 = x(2x)2 = 1.8 X 10-11 x = 1.65 X 10-4 M Sample Problem Determine the molar solubility of magnesium hydroxide. Mg(OH)2 (s) Mg2+ + 2 OHx 2x ksp= 1.8 X 10-11 Ksp = [Mg2+][OH-]2 = x(2x)2 = 1.8 X 10-11 x = 1.65 X 10-4 M 1.65 X 10-4 mole 58.31 g L Mole Mg = 9.6 X 10-6 g Mg(OH)2/L Sample Problem Determine the additional mass of magnesium hydroxide dissolved, after the addition of 125 mL of 1.00 X 10-4 M HCl. Mg(OH)2 (s) Mg2+ + 2 OH- Which way will the equilibrium shift? ksp= 1.8 X 10-11 Sample Problem Determine the additional mass of magnesium hydroxide dissolved, after the addition of 125 mL of 1.00 X 10-4 M HCl. Mg(OH)2 (s) Mg2+ + 2 OH- ksp= 1.8 X 10-11 Which way will the equilibrium shift? Right Sample Problem Determine the additional mass of magnesium hydroxide dissolved, after the addition of 125 mL of 1.00 X 10-4 M HCl. Mg(OH)2 (s) Mg2+ + 2 OH- ksp= 1.8 X 10-11 Which way will the equilibrium shift? Right What is the hydroxide ion concentration after addition of the HCl? Sample Problem Determine the additional mass of magnesium hydroxide dissolved, after the addition of 125 mL of 1.00 X 10-4 M HCl. Mg(OH)2 (s) Mg2+ + 2 OH- ksp= 1.8 X 10-11 Which way will the equilibrium shift? Right What is the hydroxide ion concentration after addition of the HCl? 2(6.8 X 10-6)Mole OH- L = 1.36 X 10-5 mole OHL 1.00 X 10-4 mole H+ 125 mL = 1.25 X 10-5 mole H+ 1000 mL Sample Problem 1.36 X 10-5 mole OH- - 1.25 X 10-5 mole H+ = 1.11 X 10-5 mole OH- Initial moles OH- Consumed OH- Excess OH- 1.1 X 10-6 mole OH = 8.8 X 10-6 M OH0.125 L What will the equilibrium do now? Shift right to produce more OH-, so that the ion product will be equal to the Ksp. [Mg2+][OH-] = 1.8 X 10-11 ½(1.36X10-5)x = 1.8X10-11 X = [OH-] = 2.65 X 10-6 Sample Problem 1.36 X 10-5 mole OH- - 1.25 X 10-5 mole H+ = 1.10 X 10-6 mole OHInitial moles OH- Consumed OH- Excess OH- 1.10 X 10-6 mole OH = 8.8 X 10-7 M OH1.250 L 2.65 X 10-6 mole OH- - 8.8 x 10-7 = 1.24 x 10-5 mole OHreplenished 1.24 x 10-6 mole OH- mole Mg(OH)2 58.33 g Mg(OH)2 = 2 mole OHmole Mg(OH)2 3.6X10-4 g of additional Mg(OH)2 dissolved About Dissolving Precipitates Precipitates form when the product of the ion concentration Q>Ksp. Since precipitates are in equilibrium with the ions that form the LeChatelier’s Principle will control the solution process. The previous sample problem demonstrated how solubility is controlled by Ph. Clearly pH is dependent upon solubility and this is a very important consideration in qualitative analysis, which is always emphasized in prelab lectures. If the procedure states acidic or basic, use litmus paper. If the procedure states just basic, then use pH paper and adjust the pH to 8. If the procedure requires a specific pH, then use pH paper. Solubility Controlling Factors Since precipitate formation is related to solubility, then the following factors control precipitation also. These factors control the process, by adding or removing ions from the equilibrium mixture according to leChateliers principle. The pH of the solution removing H+ or OH- by adding acid or base Formation of a complex Adding a substance to form a coordination compound Formation of a gas Adding a substance to convert one of the ions to a gas. Dissolving AgCl (s) One of the most common applications of precipitate control by complex formation involves the insoluble silver chloride precipitate. AgCl(s) Ag+(aq) + Cl-(aq) Ammonia will complex with silver ion to from the diamminosilver(I) complex, thus removing silver ion from solution. According to LeChatelier’s principle, silver chloride produces more silver ions which are then complexed with ammonia until all of the silver chloride solid is dissolved. The following slide illustrates this process quantitatively. Complex Formation Consider silver complex formation with ammonia Ag+ + NH3 ⇄ Ag(NH3)+ K1 = 2.1x103 Ag(NH3)+ + NH3 ⇄ Ag(NH3)2 K2 = 8.2x103 Ag+ + 2NH3 → Ag(NH3)2+ Kf = K1 x K2 Kf =1.72x107 When using a large excess of NH3 and since the formation constants are large, the reaction can be considered to be complete, since the overall constant is 1.72x107. Complex Formation Ag+ + 2NH3 → Ag(NH3)2+ Given: [Ag+]i = 5x10-4 and [NH3]I = 1.0 Then [Ag+]f = 0 and [NH3]f = 1.0- 2(5x10-4), and [Ag(NH3)2+]=5x10-4 Yes, but there is a small amount of Ag+ so then how can we calculate this concentration? Next slide! Complex Formation How much [Ag(NH3)+] is present can be calculated using the K2 K2= [Ag(NH3)2+]/[NH3][ Ag(NH3)+] Solving for [Ag(NH3)+] = [Ag(NH3)2+]/[NH3][ K2] [Ag(NH3)+]=6.1x10-8, using 1.0 for ammonia In a similar way, using K1, [Ag+] can be determined to be 2.9x10-11 Complex Formation How do you dissolve and insoluble salt? For example AgCl The equilibrium is AgCl ⇄ Ag+ + ClNeed to shift the equilibrium to the right to dissolve the solid AgCl Consider the following process AgCl ⇄ Ag+ + ClAg+ + NH3 ⇄ Ag(NH3) Ksp=1.6x10-10 K1 = 2.1x103 Ag(NH3)+ + NH3 ⇄ Ag(NH3)2+ K2 = 8.2x103 AgCl (s) + 2NH3 ⇄ Ag(NH3)2+ + Cl- K1xK2x Ksp Complex Formation 10.0 0 0 initial AgCl (s) + 2NH3 ⇄ Ag(NH3)2+ + Cl- K =2.8x103 10-2x x x Use the equilibria expression above to calculate the solubility of AgCl in a 10.0 M ammonia solution K = [Ag(NH3)2+][Cl-]/[NH3]2 → 2.8x10-3 = x2/ (10-2x)2 X=0.48 M The End