chapter15
advertisement

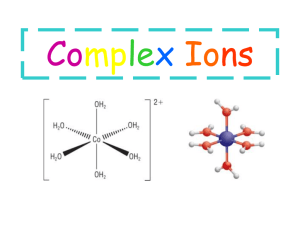
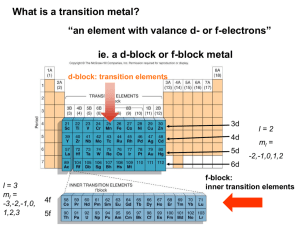
William L Masterton Cecile N. Hurley http://academic.cengage.com/chemistry/masterton Chapter 15 Complex Ions Edward J. Neth • University of Connecticut Outline 1. Composition of complex ions 2. Geometry of complex ions 3. Electronic structure of complex ions 4. Formation constants of complex ions Compounds of Transition Metals • Components • Transition metal • Associated ions and molecules • Counter-ion Components of Complex Ions and Compounds of Them • • • • Composition of complex ions Geometry of complex ions Electronic structure of central metal atom or ion Equilibrium constant for the formation of the complex ion Composition of Complex Ions • Cu2+ (aq) + 4NH3 (aq) ⇌ Cu(NH3)42+ (aq) • When ammonia is added to a solution of aqueous copper(II) ion, the color changes from pale blue to deep blue • The species that accounts for the color is a complex made up of copper(II) ion and four associated ammonia molecules The Nature of the Complex Ion, Cu(NH3)42+ • Each ammonia molecule has a lone pair on the nitrogen • A bond forms between the ammonia and the copper(II) ion • Both electrons come from ammonia • Result is a coordinate covalent bond Terminology • The species is referred to as a complex ion • Central metal ion • Small molecules or ions surround it • These are called ligands • The number of atoms bonded to the metal is the coordination number • This may or may not be equal to the number of ligands, as we will see Complex Ion or Compound? • The species, Cu(NH3)42+, is clearly a complex ion; because of the charge, it cannot be a compound • Complex ions can associate with other ions to form compounds: • [Cu(NH3)4]F2 • Two fluorides are needed to balance the positive charge on the complex • The square brackets indicate the complex ion, i.e., everything inside is part of the complex ion Cu(NH3)42+ • The central metal ion is Cu2+ • The ligands are the NH3 molecules • The coordination number is 4 Figure 15.1 The Nature of the Metal Ion • Complex ions form from • Transition metals • Main-group metals: Al, Sn, Pb • Complex ions exist in aqueous solution • Consider Zn(NO3)2 • In water, the ion exists as Zn(H2O)42+ Lewis Acid-Base Principles • Lewis bases donate lone pairs • Lewis acids accept lone pairs The Nature of the Complex Ion • Consider that hydrated metal cations are acidic • Brønsted • Zn(H2O)42+ ⇌ Zn(H2O)3(OH)+ (aq) + H+ (aq) • Lewis • The metal can accept a pair of electrons and is therefore a Lewis acid • Consider that the ligand possesses at least one lone pair • This makes the ligand a Lewis base • The complex ion can be described as the product of a Lewis acid-base reaction Lewis and Brønsted Acids and Bases • Lewis bases are also Brønsted bases • Can accept an electron pair (or pairs) • Lewis acids need not be Brønsted acids • The Lewis model broadens the definition of an acid Charges of Complexes • Charge of complex = oxidation number of central metal + charges of ligands • Platinum(II) Table 15.1 – Complexes of Pt2+ Example 15.1 Chelating Agents • Ligands • Any molecule or anion with at least one unshared pair of electrons • Some ligands have more than one pair of unshared electrons • These ligands can coordinate using multiple pairs of electrons • Ligands that can form more than one bond to a central metal ion are called chelates Two Common Chelating Ligands • Oxalate, C2O42• Ethylenediamine, H2N-CH2CH2-NH2 • Both of these ligands coordinate in two places per ligand species • Bidentate chelating ligand Figure 15.2 Geometry of Complex Ions • Review of Chapter 7 • Recall that the geometry of a molecule can be determined by the way in which the central atom coordinates with terminal atoms • For a complex ion, the geometry is determined by the shape taken by the complex, as determined by the coordination number and nature of the metal ion and ligands Coordination Number • The most common coordination number is 6 • Geometry is octahedral • Two other coordination numbers are common • 2; Geometry is linear • 4; Geometry may be square planar or tetrahedral Table 15.2 Figure 15.2 Example 15.2 Coordination Number 2 • 2-coordinate complexes are linear • CuCl2• Ag(NH3)2+ • Au(CN)2- Coordination Number 4 • Two geometries exist • Tetrahedral • Zn(NH3)42+ • CoCl42- • Square planar • Characteristic of Cu2+ and metal ions with d8 configurations (Pt2+, Ni2+) Figure 15.3 Coordination Number 6 • 6-coordinate complexes are octahedral • The six ligands are equidistant from the central metal • The octahedron can be considered a derivative of a square plane, with two ligands added, one above and one below the plane Example 15.3 Isomerism • Two or more species with the same formula, but different chemical and physical properties are called isomers • Complex ions can show several different types of isomerism • Only type to be considered here is geometric isomerism • Only the spatial orientation of ligands differs between geometric isomers Isomerism in Square Planar Complexes • Pt(NH3)2Cl2 • Can have two different isomers: cis and trans • Complexes of the form Ma2b2 will show cis-trans isomerism • a and b are different ligands Meaning of cis and trans • Cis positions are 90° apart • Trans positions are 180° apart Figure 15.4 Isomerism in Octahedral Complexes Co(NH3)4Cl2 or Ma4b2 Chelates and Isomers • In general, chelating ligands can bridge only cis positions • The bridge is not long enough to stretch across a trans position • Chelates, due to their binding in two locations, generally produce more stable complexes • Partially a result of the geometry (ring size) • Partially as a result of the nature of the bonds Figure 15.6 Figure 15.7 Physical Properties of Isomers • Note in the last figure that the color of the two complexes differ • The cis isomer is reddish-purple • The trans isomer is green • Geometric isomerism can lead to great differences in the physical and chemical properties of compounds containing complex ions Electronic Structure of Complex Ions • Crystal Field Model explains • Color of transition metal complexes • Magnetic properties of transition metal complexes • Considers the nature of the • Metal • Ligands Transition Metal Cations • In a simple transition metal cation • There are no outer s electrons • Electrons are distributed among the five d orbitals by Hund’s Rule • Recall that Hund’s rule results in the maximum number of unpaired spins • Magnetic properties depend on distribution of electrons • Diamagnetism: no unpaired electrons • Paramagnetism: unpaired electrons Iron(II) ion • Iron(II) ion has 26-2 or 24 electrons • Shorthand notation: [Ar]3d6 • Orbital diagram: • [Ar] (↑↓) (↑ ) (↑ ) (↑ ) (↑ ) • Fe2+ is paramagnetic; there are four unpaired electrons Figure 15.8 – Colors of Transition Metal Compounds Example 15.4 d-orbitals • Recall that there are five d orbitals dz2 ,dx 2 y 2 ,dxy ,dxz ,dyz • In uncomplexed metal cations, these orbitals have the same energy Figure 15.9 Octahedral Complexes • We can collect the d orbitals into two groups: • A high energy pair, the dx2-y2 and dz2 • A low energy triplet, the dxy, dxz and dyz Why the Split into Two Groups? • In the absence of any ligands, the d orbitals have the same energy • Note that the two orbitals whose energy is considered high lie on the xyz axes • Electron density in metal interacts with the electron density of ligands brought toward the metal on the axes • Note that the two orbitals whose energy is considered lower lie between the xyz axes • There is less interaction between the metal electron density and that of the ligand The Splitting Energy, Δo • The crystal field splitting energy is given the symbol Δo • The magnitude of Δo will determine the way in which the electrons fill the d orbitals in the metal ion of the complex • If Δo is large, electrons will pair in the lower energy orbitals before occupying the higher energy ones • If Δo is small, electrons will distribute themselves in all five orbitals, pairing only in cases where there are more than five electrons Figure 15.10 Figure 15.11 Electronic Arrangements in Complexes • When Δo is large, a low spin complex results • Electrons fill the lower three orbitals before occupying the upper two • When Δo is small, a high spin complex results • Electrons distribute themselves to all five orbitals by Hund’s rule Notes on High and Low Spin • For a given cation, a high spin complex will always have a larger number of unpaired electrons than a low spin complex • The value of Δo is determined by the nature of the ligand(s) • Strong field-ligands produce low spin complexes • Example: CN-, NH3 • Weak field-ligands produce high spin complexes • Example: H2O, Cl- Example 15.5 Color • Most transition metal complexes are brightly colored • Exception: those with empty d sublevels (e.g., Sc3+; those with full d sublevels (e.g., Zn2+) • The splitting of the d sublevel results in an energy difference that corresponds to the visible region of the electromagnetic spectrum • Visible light is absorbed in the transition of an electron in the ion • Some of the wavelengths of white light are removed • Complex appears colored Figure 15.12 Titanium(III) • Consider Ti3+ in a complex • There is only one d electron • Because there is only one possible electronic transition, it is possible to calculate Δo for this ion • The ion absorbs at 510 nm (green) • The complex appears as the color complement of green (i.e., red-violet or purple) Calculating Δo for Titanium(III) E hc 3.90 10 19 J 23 1 kJ 6 . 022 X 10 E 3.90 10 19 J 1000J mol • By knowing the energy of the light absorbed, it is possible to calculate the value of Δo • For ions with more than one d electron, calculating the energy Δo is more difficult Δo and Wavelength • The smaller Δo is, the longer the wavelength of absorption • Small Δo stem from weak field ligands • Large Δo stem from strong field ligands Light Absorption and Color The Spectrochemical Series of Ligands • CN- > NO2- > en > NH3 > NCS- > H2O > F- > ClStrong field weak field Equilibrium and Complex Ions • We can consider the formation of a complex ion in aqueous solution as an equilibrium between • The reactants • Bare metal ion and ligands • The product • The complex ion • As with acid-base and gas phase equilibria, an expression for K can be written Formation Constants • Consider • Cu2+ (aq) + 4NH3 (aq) ⇌ Cu(NH3)42+ (aq) • We can write the expression for Kf (the formation constant for the ion: 2 [Cu(NH3 )4 ] Kf [Cu2 ][NH3 ]4 • In many cases, Kf is a very large number, indicating that the complex which forms is very stable Table 15.4 Example 15.6 Example 15.6 (Cont’d) Practical Example • Most cleaning compounds containing ammonia state that the product is not to be used on objects made of silver • Consider the Kf for Ag(NH3)2+: 1.7 X 107 • The complex of silver with ammonia is a very stable one • Using an ammonia-based cleaner on silver will partially dissolve the silver Chelates in Nature • Chelating agents are abundant in nature • Iron is complexed with the chelate heme • EDTA is used to complex metal ions • Treat heavy metal poisoning • Mask metal ions that promote food spoilage Heme EDTA Key Concepts 1. Relate the composition of a complex ion to its charge, coordination number, and the oxidation number of the central metal 2. Sketch the geometry of a complex ion and identify geometric isomers 3. Give the electron configuration and the orbital diagram for the transition metal at the center of the complex 4. Derive the orbital diagram (high or low spin) for the complex 5. Relate the formation equilibrium constant to the concentrations of metal ion, ligands and complex ion