Ch 23
advertisement

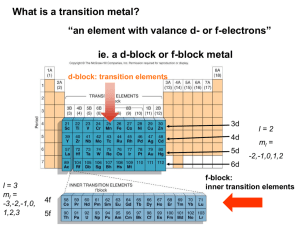
Ch 22 complex ions • Composition of the complex and nomenclature • Geometry of complex ions and isomers • Electronic structure of complex ions • Formation constant Coordination complex is the product of a Lewis acid-base reaction in which neutral molecules or anions (called ligands) bond to a central metal atom (or ion) by coordinate covalent bonds. Ligands are Lewis bases - they contain at least one pair of electrons to donate to a metal atom/ion. Ligands are also called complexing agents. Metal atoms/ions are Lewis acids - they can accept pairs of electrons from Lewis bases. Within a ligand, the atom that is directly bonded to the metal atom/ion is called the donor atom. If the coordination complex carries a net charge, the complex is called a complex ion. Compounds that contain a coordination complex are called coordination compounds. Ch 23 – Components of a coordination compound. Models wedge diagrams Formulas Structures of Complex Ions: Coordination Numbers, Geometries, and Ligands • • Coordination Number - the number of ligand atoms that are bonded directly to the central metal ion. The coordination number is specific for a given metal ion in a particular oxidation state and compound. Geometry - the geometry (shape) of a complex ion depends on the coordination number and nature of the metal ion. Coordination number 2 4 • Donor atoms per ligand - molecules and/or anions with one or more donor atoms that each donate a lone pair of electrons to the metal ion to form a covalent bond. 4 6 Shape linear Square planar Tetrahedral Octahedral Geometry Example [CuCl2][Ag(NH3)2]+ [AuCl2]- [Ni(CN)4]2[Pd(Cl)4]2[Cu(NH3)4]2+ [Pt(Cl)4]2[Cu(CN)4]3[Zn(NH3)4]2+ [Cd(Cl)4]2[Mn(Cl)4]2[Ti(H2O)6]2+ [V(CN)6]4[Cr(NH3)4Cl2]+ [FeCl6]3[Co(en)3]3+ Common ligands in coordination compounds Ligand type Example Monodentate H2O (water) F(Foloride ion) CN(cyanide ion) OHhydroxide NH3 (Amomina) Cl(Chloride ion) [S=C=N]thiocyanate ion [O=N=O]nitride ion Bidentate ethylenediamine Oxalate Polydentate diethylenetriamine Name of common ions Netrual Anionic Metal ions in complex anion Formula Name Formula Name Formula Name H2O Aqua F- Fluoro Fe (iron) Ferrate NH3 Amine Cl- Chloro Cu (copper) Cuprate CO Carbonyl Br- Bromo Pb (lead) Plumbate NO Nitrosyl I- Iodo Ag (silver) Argentate OH- Hydroxo Au (gold) Aurate CN- Cyano Sn (Tin) Stannate Formulas and Names of Coordination Compounds Rules for naming complexes: 1. The cation is named before the anion. 2. Within the complex ion, the ligands are named, in alphabetical order, before the metal ion. 3. Neutral ligands generally have the molecule name, but there are a few exceptions. Anionic ligands drop the -ide and add -o after the root name. 4. A numerical prefix indicates the number of ligands of a particular type. 5. The oxidation state of the central metal ion is given by a Roman numeral (in parentheses). 6. If the complex ion is an anion we drop the ending of the metal name and add -ate. Sample Problem Writing Names and Formulas of Coordination Compounds PROBLEM: (a) What is the systematic name of Na3[AlF6]? (b) What is the systematic name of [Co(en)2Cl2]NO3? (c) What is the formula of tetraaminebromochloroplatinum(IV) chloride? (d) What is the formula of hexaaminecobalt(III) tetrachloro-ferrate(III)? PLAN: Use the rules presented. SOLUTION: (a) The complex ion is [AlF6]3-. Six (hexa-) fluorines (fluoro-) are the ligands - hexafluoro Aluminum is the central metal atom - aluminate Aluminum has only the +3 ion so we don’t need Roman numerals. sodium hexafluoroaluminate continued Writing Names and Formulas of Coordination Compounds (b) [Co(en)2Cl2]NO3 There are two ligands, chlorine and ethylenediamine - dichloro, bis(ethylenediamine) The complex is the cation and we have to use Roman numerals for the cobalt oxidation state since it has more than one - (III) The anion, nitrate, is named last. (c) dichlorobis(ethylenediamine)cobalt(III) nitrate Tetraamine bromo chloro 4 NH3 Br- Cl- platinum (IV) chloride Pt4+ Cl- [Pt(NH3)4BrCl]Cl2 (d) Hexaamine cobalt(III) tetrachloro-ferrate(III) 6 NH3 Co3+ 4 ClFe3+ [Co(NH3)6][Cl4Fe]3 Important types of isomerism in coordination compounds. ISOMERS Same chemical formula, but different properties Constitutional (structural) isomers Stereoisomers Atoms connected differently Different spatial arrangement Coordination isomers Ligand and counter-ion exchange Linkage isomers Different donor atom Geometric (cis-trans) isomers (diastereomers) Different arrangement around metal ion Optical isomers (enantiomers) Nonsuperimposable mirror images Sample Problem Determining the Type of Stereoisomerism PROBLEM: Draw all stereoisomers for each of the following and state the type of isomerism: (b) [Cr(en)3]3+ (en = H2NCH2CH2NH2) (a) [Pt(NH ) Br ] 3 2 PLAN: 2 Determine the geometry around each metal ion and the nature of the ligands. Place the ligands in as many different positions as possible. Look for cis-trans and optical isomers. SOLUTION: (a) Pt(II) forms a square planar complex and there are two pair of monodentate ligands - NH3 and Br. Br NH3 H 3N Pt H 3N Pt Br trans Br H 3N Br cis These are geometric isomers; they are not optical isomers since they are superimposable on their mirror images. continued Determining the Type of Stereoisomerism (b) Ethylenediamine is a bidentate ligand. Cr3+ is hexacoordinated and will form an octahedral geometry. Since all of the ligands are identical, there will be no geometric isomerism possible. 3+ 3+ N N N N N N Cr N Cr N N N N N rotate 3+ N N N Cr N N N The mirror images are nonsuperimposable and are therefore optical isomers. Linkage isomers Geometric (cis-trans) isomerism. Optical isomerism in an octahedral complex ion. Colors of representative compounds of the Period 4 transition metals. nickel(II) nitrate hexahydrate sodium chromate titanium oxide scandium oxide vanadyl sulfate dihydrate potassium ferricyanide manganese(II) chloride tetrahydrate cobalt(II) chloride hexahydrate zinc sulfate heptahydrate copper(II) sulfate pentahydrate Important types of isomerism in coordination compounds. ISOMERS Same chemical formula, but different properties Constitutional (structural) isomers Stereoisomers Atoms connected differently Different spatial arrangement Linkage isomers Coordination isomers Ligand and counter-ion exchange NO2- is the counter ion [Pt(NH3)4Cl2](NO2)2 [Pt(NH3)4NO2]Cl2 Optical isomers (enantiomers) Geometric (cis-trans) isomers Nonsuperimposable (diastereomers) mirror images Different [Co(NH3)5(NO2)] Cl arrangement around [Co(NH3)5(ONO)] Cl metal ion Different donor atom Stereoisomers Geometric (cis-trans) isomers (diastereomers) Different arrangement around metal ion Diastereomers Cis – the identical ligands next to each other Trans-the identical lagands cross from each other Optical isomers (enantiomers) Nonsuperimposable mirror images 3+ 3+ N N N N N Cr N N Cr N N N Cisplatin effective antitumor agent. Cisplatin may work by Lying within the cancer cell’s DNA double helix to prevent the DNA duplication. Transplatin has no effect on tumor. N N An artist’s wheel. Hybrid orbitals and bonding in the tetrahedral [Zn(OH)4]2- ion. Color absorbed Color transmitted (Complementary) Wavelength (nm) Red Green-blue 750-610 Orange Blue- green 610-595 Yellow Violet 595-580 Green Red-violet 580-500 Blue Orange-yellow 500-435 Violet Yellow 435-380 The color of [Ti(H2O)6]3+. [V(H2O)6]2+ [V(H2O)6]3+ [Cr(NH3)6]3+ [Cr(NH3)5Cl ]2+ The spectrochemical series. • • For a given ligand, the color depends on the oxidation state of the metal ion. For a given metal ion, the color depends on the ligand. Effects of the metal oxidation state and of ligand identity on color. The configuration of central metal elements Finding the Number of Unpaired Electrons PROBLEM: PLAN: The alloy SmCo5 forms a permanent magnet because both samarium and cobalt have unpaired electrons. How many unpaired electrons are in the Sm atom (Z = 62)? Write the condensed configuration of Sm and, using Hund’s rule and the aufbau principle, place electrons into a partial orbital diagram. SOLUTION: Sm is the eighth element after Xe. Two electrons go into the 6s sublevel and the remaining six electrons into the 4f (which fills before the 5d). Sm is [Xe]6s 2 4f 6 6s2 4f6 There are 6 unpaired e- in Sm. 5d0 Crystal Field Theory Splitting of d-orbital energies by an octahedral field of ligands. D is the splitting energy The effect of ligand on splitting energy. The spectrochemical series. • For a given ligand, the color depends on the oxidation state of the metal ion. • For a given metal ion, the color depends on the ligand. I- < Cl- < F- < OH- < H2O < SCN- < NH3 < en < NO2- < CN- < CO WEAKER FIELD Splitting Energy : D Wavelength: STRONGER FIELD SMALLER D LARGER D LONGER SHORTER HIGH SPIN LOW SPIN Ranking Crystal Field Splitting Energies for Complex Ions of a Given Metal PROBLEM: Rank the ions [Ti(H2O)6]3+, [Ti(NH3)6]3+, and [Ti(CN)6]3- in terms of the relative value of D and of the energy of visible light absorbed. PLAN: The oxidation state of Ti is 3+ in all of the complexes so we are looking at the crystal field strength of the ligands. The stronger the ligand the greater the splitting and the higher the energy of the light absorbed. SOLUTION: The field strength according to is CN- > NH3 > H2O. So the relative values of D and energy of light absorbed will be [Ti(CN)6]3- > [Ti(NH3)6]3+ > [Ti(H2O)6]3+ Hybrid orbitals and bonding in the octahedral [Cr(NH3)6]3+ ion. Cr Hybrid orbitals and bonding in the square planar [Ni(CN)4]2- ion. Ni Identifying Complex Ions as High Spin or Low Spin – number of electrons unpaired PROBLEM: Iron (II) forms an essential complex in hemoglobin. For each of the two octahedral complex ions [Fe(H2O)6]2+ and [Fe(CN)6]4-, draw an orbital splitting diagram, predict the number of unpaired electrons, and identify the ion as low or high spin. PLAN: The electron configuration of Fe2+ gives us information that the iron has 6d electrons. The two ligands have field strengths. Draw the orbital box diagrams, splitting the d orbitals into eg and t2g. Add the electrons noting that a weak-field ligand gives the maximum number of unpaired electrons and a high-spin complex and vice-versa. potential energy SOLUTION: [Fe(CN)6]4- ]2+ [Fe(H2O)6 4 unpaired e-(high spin) eg t2g eg no unpaired e-(low spin) t2g High-spin and low-spin complex ions of Mn2+. Orbital occupancy for high- and low-spin complexes of d4 through d7 metal ions. high spin: weak-field ligand low spin: strong-field ligand high spin: weak-field ligand low spin: strong-field ligand Formation constant of complex ions The equilibrium constant for the formation of a complex ion is called formation constant, Kf. A complex formed by Ca with EDTA is used to treat the Pb poisoning. * * Ca2+ + EDTA Ca(EDTA)2+ * * * Ethylenediaminetetraacetic * * * EDTA titration curve Calculate the shape of the titration curve for the reaction of 50.00 ml of 0.0400 M Ca2+ with the 0.0800 M EDTA. (Buffered to 10.00, Kf = 4.91010) Free concentration of metal ions as a function of EDTA additionn volume Ca2+ 1. + EDTA CaY2- Find the V EDTA at equivalence point : [Ca2+]=[EDTA] 50.00 ml 0.0400 M = V 0.0800 M V=25.00 ml 2. Before equivalence point : Consider addition of 5 ml EDTA Total volume of solution is 50 ml + 5 ml = 55 ml Ca2+ I C E + 50.00 ml 0.0400 -x 2mmol-x EDTA 0 5 ml 0.08 M CaY20 +x 0.4 mmol = x Total volume V= 50.00 ml + 5 ml = 55 ml Unreacted Ca2+ : (50.00 ml 0.0400 M – 5 ml 0.08 M)/55 ml = 0.029 M p[Ca2+] = -log[Ca2+] = 1.54 Calculate pCa2+ when adding EDTA 10 ml, 15ml and 20 ml 3. At equivalence point: Ca2+ I C E • • • x + EDTA x CaY20.0267 0.0267-x [CaY2-] = [Ca2+]initial = [Y4-]initial = 0.0400 M 50 ml / (50 + 25) ml =0.0267 M Kf ’ = Kf = [CaY2-]/[Ca2+][EDTA] = 4.9 1010 0.36 = 1.8 1010 x = 1.2 10-6 pCa2+ = -log 1.2 10-6 = 5.91 4. After equivalence point : After equivalence point, there is excess EDTA. The concentration of CaY2- and excess EDTA can be calculate and then from Kf to figure out Ca2+ free ions concentration. After equivalence point : Consider 26 ml of EDTA [EDTA] = 0.0800 M (26-25)ml / (26+50) ml = 1.05 10 -3 [CaY2-] = 0.0400 M 50 ml / (26+50) ml = 2.63 10 -3 Kf ’ = [CaY2-] / [Ca2+][EDTA] = 1.8 1010 [Ca2+] = 1.8 10-9 pCa2+ = 8.86 Calculate two more points Questions for extra credit 1. Define the spectrochemical series and classify the order of the following ligand strength. I- , Cl- , F- , OH- , H2O , SCN- , NH3 , en , NO2- , CN- , CO 2. Draw the hybrid orbitals and bonding in the octahedral [Cr(NH3)6]3+ ion. 3. Draw the hybrid orbitals and bonding in the square planar [Ni(CN)4]2- ion. 4. For each of the two octahedral complex ions [Fe(H2O)6]2+ and [Fe(CN)6]4-, draw an orbital splitting diagram, predict the number of unpaired electrons, and identify the ion as low or high spin. 5. Explain why The alloy SmCo5 forms a permanent magnet. 6. Draw the electronic configuration diagram of Sm. 7. Rank the ions [Ti(H2O)6]3+, [Ti(NH3)6]3+, and [Ti(CN)6]3- in terms of the relative value of D and of the energy of visible light absorbed. 1. Determine the shape and draw the geometry. Give two examples. CN 2 4 4 6 Shape Geometry Example Coordination number = CN Extra credit 2. Name the follow compounds. Color absorbed Color transmitted (Complementary) Wavelength (nm) Red Green-blue 750-610 • the systematic name of Na3[AlF6] • systematic name of [Co(en)2Cl2]NO3 Orange Blue- green 610-595 • systematic name of[Pt(NH3)4BrCl]Cl2 Yellow Violet 595-580 • systematic name of[Co(NH3)6][Cl4Fe]3 Green Red-violet 580-500 Blue Orange-yellow 500-435 Violet Yellow 435-380 3. How many types of isomerism in coordination compounds. 4. Draw all stereoisomers for each of the following and state the type of isomerism: • [Pt(NH3)2Br2] • [Cr(en)3]3+ (en = H2NCH2CH2NH2) 5. Tabulate the color absorbed and the color we can identify by our eyes. List the wavelength associated.