Spectroscopy
advertisement

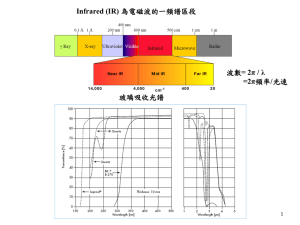
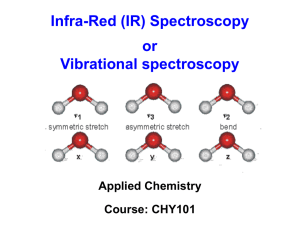
INTRODUCTION TO SPECTROSCOPY Spectroscopy Spectroscopy is a general term referring to the interactions of various types of electromagnetic radiation with matter. Exactly how the radiation interacts with matter is directly dependent on the energy of the radiation. Spectroscopy The higher energy ultraviolet and visible wavelengths affect the energy levels of the outer electrons. Infrared radiation is absorbed by matter resulting in rotation and/or vibration of molecules. Radio waves are used in nuclear magnetic Resonance and affect the spin of nuclei in a magnetic field. THE ELECTROMAGNETIC SPECTRUM Important: As the wavelength gets shorter, the energy of the radiation increases. PARTICLE NATURE OF RADIATION Electromagnetic radiation is also described as having the properties of particles. Molecules exist in a certain number of possible states corresponding to definite amounts of energy. Molecules can absorb energy and change to a higher energy level called the excited state. The amount of energy absorbed in this transition is exactly equal to the energy difference between the states. Energy Level Diagram for an Atom of Sodium E0 Ground State 330 nm E1 590 nm E2 Energy Level Diagram for a Simple Molecule E2 Relaxation from the E2 energy state to E0 may go to different vibrational energy states, emitting different wavelengths. E1 Excitation to the next electronic energy level caused by adsorption of specific wavelengths E0 Ground State e4 e3 e2 e1 Vibrational Energy Levels UV/VIS SPECTROSCOPY Visible (380-780 nanometers) Ultraviolet (UV) (10 – 380 nanometers). Below about 200 nm, air absorbs the UV light and instruments must be operated under a vacuum How many µm is 780 nanometers? What is the corresponding wave number? Absorption of ultraviolet and visible light only takes place in molecules with valence electrons of low excitation energy. bonding p antibonding p transitions have high molar absorbtivities Energy Antibonding s Antibonding p e Non-bonding e Bonding p e e Bonding s Absorbs below 200 nm not seen in typical UV spectra Wavelengths Absorbed by Functional Groups Again, demonstrates the moieties contributing to absorbance from 200-800 nm, because pi electron functions and atoms having no bonding valence shell electron pairs. Wavelengths Absorbed by Functional Groups What is the absorbance max? Example of a Method to Determine the Absorption Spectra of an Organic Compound Woodward’s Rules For Conjugated Carbonyl Compounds Aldehyde: 208 nm Extended conjugation: 30 nm Homodiene component: 39 nm a-Alkyl groups or ring residues: 10 nm d-Alkyl groups or ring residues: 18 nm Calculated: 304 nm OTHER CONCEPTS IMPORTANT UV/VIS SPECTROSCOPY TO UV/Vis spectra can be used to some extent for compound identification, however, many compounds have similar spectra. Solvents can cause a shift in the absorbed wavelengths. Therefore, the same solvent must be used when comparing absorbance spectra for identification purposes. Many inorganic species also absorb energy in the UV/Vis region of the spectrum. INFRARED SPECTROSCOPY Absorption of electromagnetic energy in the infrared region causes changes in the vibrational energy of molecules Energy changes are typically 6000 to 42,000 J/mol which corresponds to wavelengths of 2.5-40 mm (250-4000/cm) http://sis.bris.ac.uk/~sd9319/spec/IR.htm Many of these bands can be assigned to the vibration of particular chemical groups in the molecule Absorption Frequencies of Functional Groups See Appendix B (2 tables) and Table 2 1 C5H10O 3400-3200 cm-1: no OH or NH present 3100 cm-1: no peak to suggest unsaturated CH 2900 cm-1: strong peak indicating saturated CH 2200 cm-1: no unsymmetrical triple bonds Structure: IUPAC Name: 3-pentanone 1710 cm-1: strong carbonyl absorbance 1610 cm-1: no absorbance to suggest carbon-carbon double bonds 2 C8H8O 3400-3200 cm-1: no OH or NH present 3100 cm-1: moderate peak suggesting unsaturated CH 2900 cm-1: weak peak indicating possible saturated CH Structure: 2200 cm-1: no unsymmetrical triple bonds IUPAC Name: acetophenone 1690 cm-1: strong carbonyl absorbance 1610 cm-1: weak absorbance bands consistent with carbon-carbon double bonds 3 C7H8O 3400-3200 cm-1: strong peak indicating OH is present 3100 cm-1: weak peak suggesting possible unsaturated CH 2900 cm-1: weak peak indicating possible saturated CH 2200 cm-1: no unsymmetrical triple bonds 1720 cm-1: no carbonyl absorbance 1450-1500 cm-1: moderate absorbance bands consistent with aromatic carbon-carbon double bonds Structure: IUPAC Name: benzyl alcohol 4 C7H6O 3400-3200 cm-1: no peak which would indicate OH or NH 3100 cm-1: moderate peak indicating unsaturated CH 2900 cm-1: no peaks to indicate saturated CH Structure: 2750-2600 cm-1 ; moderate peaks strongly suggesting aldehydic CH IUPAC Name: benzaldehyde 2250 cm-1: no absorbance indicating an unsymmetrical triple bonds 1700 cm-1: strong carbonyl absorbance 1450-1600 cm-1 :moderate absorbance bands consistent with aromatic carbon-carbon double bonds 5 C3H10NO 3400-3200 cm-1: strong peak suggesting OH or NH 3100 cm-1: minor peak indicating possible unsaturated CH 2900 cm-1: minor peaks indicating saturated CH 2200 cm-1: no unsymmetrical triple bonds 1650 cm-1: strong carbonyl absorbance 1550 cm-1: moderate absorbance band, characteristic of ‘N-H bending’ Structure: IUPAC Name: N-methylacetamide 6 C4H8O2 3400-3200 cm-1: no peak to indicate an OH or NH 3100 cm-1: no peak to indicate unsaturated CH 2900 cm-1: minor peaks indicating saturated CH 2200 cm-1: no unsymmetrical triple bonds 1760 cm-1: strong carbonyl absorbance Structure: IUPAC Name: ethyl acetate 1600 cm-1: no peak to indicate a carbon-carbon double bond 1250 cm-1: strong, broad peak consistent with a carbon-oxygen single bond C 4 H 8O 2 NMR SPECTROSCOPY NMR SPECTROSCOPY Nuclear magnetic resonance spectrometry (NMR) is based on the absorption of electromagnetic radiation in the radio-frequency region of the spectrum resulting in changes in the orientation of spinning nuclei in a magnetic field NMR Energies 0.1 J/mol IR Energies 6000 to 42,000 J/mol UV/Vis Energies >100,000 J/mol As the nucleus spins it produces a magnetic moment or dipole along the axis. The relative values of the magnetic moment and the angular momentum determine the frequency at which energy can be absorbed. Relative Sensitivity of NMR Techniques Table 4. Nuclear Spin Quantum Numbers and Magnetic Properties of Nuclei Nucleus Nuclear spin Quantum Number Hydrogen Deuterium Carbon 12 Carbon 13 Fluorine 19 1/2 1 0 1/2 1/2 Magnetic Moment, (ampere square meter x 1027 14.09 4.34 -3.52 13.28 Resonance Frequency in MHz at 1.4092 TESLA 60.000 9.211 -15.085 56.446 Relative Sensitivity at the Natural Isotopic Abundance 1.00 0.00015 -0.00018 0.834 Proton Magnetic Resonance In PMR the instrument is detecting the energy difference between protons with a spin of +1/2 (low energy) and -1/2 higher energy. The application of electromagnetic radiation can excite the nuclei into the higher energy level. The frequency that causes the excitation is determined by the difference in energy between the energy levels. Chemical Shift The NMR spectrum arises because nuclei in different parts of the molecule experience different local magnetic fields according to the molecular structure, and so have different frequencies at which they absorb. This difference is called the chemical shift. This is because the nucleus is shielded from this field to a greater or lesser extent by the other atoms in the vicinity and their electrons. Benzene, C6H6 has only one sort of hydrogen atom, so that the NMR spectrum shows a single peak (the TMS peak is omitted): Ethanal CH3CHO has two sorts of hydrogen atom, those on the methyl group and the one on the aldehyde group. It therefore has two peaks in its spectrum (the TMS peak is omitted). Ethanol CH3CH2OH has methyl hydrogen, methylene hydrogen, and hydroxyl hydrogen. It therefore has three peaks in its spectrum Spin-spin coupling In ethanol, the hydrogen atoms on the methyl group interact with those on the methylene group – their magnetic fields couple. The effect of coupling on the spectrum is that the lines are split into multiplets. Most coupling occurs between hydrogen atoms on adjacent carbon atoms, so in the ethanol spectrum there is splitting of the lines due to the methyl and methylene hydrogen atoms, but not that of the hydroxyl hydrogen – it is too far away.