Al_Benzene Reishus OH 2013
advertisement

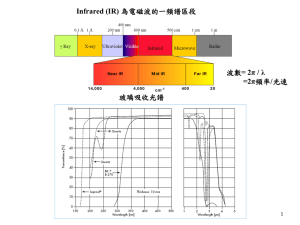
Infrared Photodissociation Spectroscopy of Aluminum Benzene Cation Complexes Nicki Reishus, Biswajit Bandyopadhyay and Michael A. Duncan Department of Chemistry, University of Georgia, Athens, GA 30602 www.arches.uga.edu/~maduncan/ nreishus@uga.edu; maduncan@uga.edu Metal benzene sandwiches • 1973 Nobel to Wilkinson and Fischer for work on organometallic sandwiches1 Ferrocene Wilkinson and Woodward 1952 Di-benzene chromium E. O. Fischer 1955 1. Fischer, E. O.; Hafner, W. Z. Naturforsch. 1955, 10b, 665. Previous work on metal-benzene ions: • Duncan group electronic photodissociation1 • Kaya and coworkers multiple-decker sandwiches and photoelectron spectroscopy (PES) on anions2 • Lisy and coworkers infrared (IR) spectroscopy in C–H stretch region with alkali metals3 • Duncan et al. FELIX in far IR and IR-OPO in mid IR on transition metals4 • D. S. Yang group ZEKE spectroscopy on transition metals5 1. 2. 3. 4. 5. Willey, K. F.; Yeh, C. S.; Robbins, D. L.; Duncan, M. A., J. Phys. Chem. 1992, 96, 9106-9111. Nakajima, A.; Kaya, K., J. Phys. Chem. A 2000, 104, 176-1913. Cabarcos, O. M.; Weinheimer, C. J.; Lisy, J. M., J. Chem. Phys. 1999, 110, 8429-8435. M.A. Duncan, Int. J. Mass Spectrom. 2008, 272, 99. B. R. Sohnlein, Y. Lei and D.-S. Yang, J. Chem. Phys. 2007, 127, 114302/1-114302/10. Previous work: OPO/OPA ν +ν 19 8 ν20 • OPO/OPA with argon tagging used for C–H stretch region • Free benzene Fermi resonance1: 3048, 3079, 3101 cm-1 ν1+ν6+ ν19 + V (bz)3 + V (bz)2Ar • V+(bz)3 no Ar tagging needed, and free benzene Fermi resonance observed • 3rd + V (bz)Ar benzene is external 2700 2800 2900 3000 3100 3200 3300 -1 cm Jaeger, T. D.; Pillai, E. D.; Duncan, M. A., J. Phys. Chem. A 2004, 108, 6605-6610. 1. Snavely, D. L.; Walters, V.A.; Colson, S.D.; Wiberg, K. B., Chem. Phys. Lett. 1984, 103, 423-429. Experimental • OPO/OPA range: 600-4500 cm-1 • Binding energies Al+(bz) = 35.2 kcal/mol1, Al+(bz)Ar = 0.8 kcal/mol (MP2/6-311+G** ) • Theory: B3LYP/6-311+G** Aluminum benzene mass spec: 1. Dunbar, R. C.; Klippenstein, S. J.; Hrusak, J.; Stockigt, D.; Schwarz, H. J. Am. Chem. Soc. 1996, 118, 5277-5283. Al+(bz)Ar 673 • 750 ν11 oop H-bend, 77 blue shift • 990 cm-1 ν1 sym. C stretch, (not IR active in free benzene) cm-1 cm-1 • 1476 cm-1 ν19 in–plane C ring distortion, indicator of charge transfer1, 10 cm-1 red shift • 1643 cm-1 ν8 C ring stretch (not IR active in free benzene), 33 cm-1 blue shift • 3033 cm-1 ν20 C–H stretch • 3097, 3065 cm-1 Fermi resonance: ν20 C–H stretch & ν8+ν19, ν1+ν6+ ν19, respectively • 730 993 1610 1481 990 1610 3079 3048 3101 3121 737 1479 Theory scaled for each mode 981 1. Chaquin, P.; Costa, D.; Lepetit, C.; Che, M. J. Phys. Chem. A 2001 105, 4541-4545. 1486 van Heijnsbergen, D.; Jaeger, T. D.; von Helden, G.; Meijer, G.; Duncan, M. A., Chem. Phys. Lett. 2002, 364, 345-351. Al+(bz)2Ar • 3079 Fermi resonance caused by addition of second benzene • 1596 cm-1 ν8 ring stretch, goes from 33 cm-1 blue shift to 14 cm-1 red shift • 1477 cm-1 ν19 in–plane C ring distortion, 9 cm-1 red shift • 719 cm-1 ν11 oop H-bend, 46 cm-1 blue shift, 31 cm-1 less than Al+(bz)Ar • Bond distance 2.5 Å 2.8 Å cm-1 673 1486 1610 993 cm-1 3079 3048 3101 Where does a 2nd benzene go? • Al+ 3s2 • s orbital polarizable1 • 1st ligand polarizes s orbital1 e- e- Walters, R. S.; Brinkmann, N. R.; Schaefer, H. F.; Duncan, M. A., J. Phys. Chem. A 2003, 107, 7396-7405. 1. Bauschlicher, Jr., C. W.; Partridges, H. J. Phys. Chem. 1991, 95, 9694-9698. Al+(bz)3Ar • Fourth Fermi resonance disappears • 1643 cm-1 ν8 ring stretch goes away • 1478 cm-1 ν19 in–plane C ring distortion, 8 cm-1 red shift • 723 cm-1 ν11 oop H-bend, 50 cm-1 blue shift, 4 cm-1 blue shift from Al+(bz)2Ar • Theory indicates ν11 red shifts from Al+(bz)Ar • Bond distance increases to 2.9 Å 673 993 1486 1610 ? 3079 3101 3048 Vib. of external ligands are usually un-shifted • • External ligands cause un-shifted ligand peaks to appear Free CO2 band Coordinated CO2 band But there is no evidence in Al+(bz)3Ar for un-shifted bands Ricks, A. M.; Brathwaite, A. D.; Duncan, M. A. J. of Phys. Chem. A 2013 117 , 1001-1010. Al+(bz)4 • 1481 cm-1 ν19 in–plane C ring distortion, 5 cm-1 red shift • Theory predicts a wider ν11 peak due to a 4th external benzene • No ν11 cm-1 peak observed (likely because of diss. energy) • Again no evidence of un-shifted bands 673 993 ? • Different IR intensities for bonded vs external bands? 1486 1610 3079 3048 3101 Spectra of Al+(bz)1-3Ar, Al+(bz)4 do not show evidence for an external benzene ? ? cm-1 Conclusions • Best quality IR spectra yet measured for a metal ion benzene system • ν19 shows there is not much charge transfer between the Al+ and benzene • Theory predicts a consistent red shift for the ~700 cm-1 band, but experiments show a change in relative shift from red to blue • Theory does not predict the ν8 band • Coordination is not obvious from spectra • Theory shows a coordination of three Binding energies: theory Literature1 Binding energies B3LYP/ of ligand 6-311+G** (kcal/mol) MP2/ B97D/ 6-311+G** 6-311+G** Al+-Benzene 30.0 35.4 34.1 35 ±2 Al+-(Benzene)2 11.6 - 19.6 - Al+-(Benzene)3 4.5 - - - Al+-(Benzene)4 2.8 - - - 1. Dunbar, R. C.; Klippenstein, S. J.; Hrusak, J.; Stockigt, D.; Schwarz, H. J. Am. Chem. Soc. 1996, 118, 5277-5283.