+1/2
advertisement

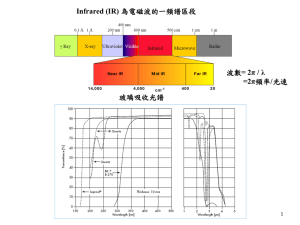
Spectroscopy “seeing the unseeable” Color 400 500 600 l, nm Violet 400-420 Indigo 420-440 Blue 440-490 Green 490-570 Yellow 570-585 Orange 585-620 Red 620-780 700 800 “color wheel” b-carotene b-carotene, lmax = 455 nm lmax is at 455 – in the far blue region of the spectrum – this is absorbed The remaining light has the complementary color of orange electromagnetic relationships: λυ = c λ 1/υ E = hυ E υ E = hc/λ E 1/λ λ = wave length υ = frequency c = speed of light E = kinetic energy h = Planck’s constant λ c Two oscillators will strongly interact when their energies are equal. E1 = E2 λ1 = λ2 υ1 = υ2 If the energies are different, they will not strongly interact! We can use electromagnetic radiation to probe atoms and molecules to find what energies they contain. Spectroscopy The higher energy ultraviolet and visible wavelengths affect the energy levels of the outer electrons. Infrared radiation is absorbed by matter resulting in rotation and/or vibration of molecules. Radio waves are used in nuclear magnetic Resonance and affect the spin of nuclei in a magnetic field. Energy Level Diagram for an Atom of Sodium E0 Ground State 330 nm E1 590 nm E2 Energy Level Diagram for a Simple Molecule E2 Relaxation from the E2 energy state to E0 may go to different vibrational energy states, emitting different wavelengths. E1 Excitation to the next electronic energy level caused by adsorption of specific wavelengths E0 Ground State e4 e3 e2 e1 Vibrational Energy Levels Molecular Orbital's Orbitals Stable Energy to Excited Energy LUMO: Lowest Unoccupied Molecular Orbital HOMO: Highest Occupied Molecular Orbital UV/VIS SPECTROSCOPY Visible (380-780 nanometers) Ultraviolet (UV) (10 – 380 nanometers). Below about 200 nm, air absorbs the UV light and instruments must be operated under a vacuum The Spectroscopic Process Instrumentation monochromator/ beam splitter optics I0 I0 I log(I0/I) = A detector UV-VIS sources sample Deuterium lamp – covers the UV – 200-330 Tungsten lamp – covers 330-700 reference • • I0 200 l, nm 700 UV Spectroscopy_DAD A recent improvement is the diode-array spectrophotometer here a prism (dispersion device) breaks apart the full spectrum transmitted through the sample Each individual band of UV is detected by a individual diodes on a silicon wafer simultaneously – the obvious limitation is the size of the diode, so some loss of resolution over traditional instruments is observed Diode array sample UV-VIS sources Polychromator – entrance slit and dispersion device UV Spectroscopy 1. Only quartz is transparent in the full 200-700 nm range; plastic and glass are only suitable for visible spectra 2. Concentration is empirically determined A typical sample cell (commonly called a cuvette): Wavelengths Absorbed by Functional Groups Wavelengths Absorbed by Functional Groups Example of a Method to Determine the Absorption Spectra of an Organic Compound Woodward’s Rules For Conjugated Carbonyl Compounds Aldehyde: 208 nm Extended conjugation: 30 nm Homodiene component: 39 nm a-Alkyl groups or ring residues: 10 nm d-Alkyl groups or ring residues: 18 nm Calculated: 304 nm OTHER CONCEPTS IMPORTANT TO UV/VIS SPECTROSCOPY UV/Vis spectra can be used to some extent for compound identification, however, many compounds have similar spectra. Solvents can cause a shift in the absorbed wavelengths. Therefore, the same solvent must be used when comparing absorbance spectra for identification purposes. Many inorganic species also absorb energy in the UV/Vis region of the spectrum. UV Spectroscopy Solvents must be transparent in the region to be observed; the wavelength where a solvent is no longer transparent is referred to as the cutoff Since spectra are only obtained up to 200 nm, solvents typically only need to lack conjugated systems or carbonyls Common solvents and cutoffs: acetonitrile 190 chloroform 240 cyclohexane 195 1,4-dioxane 215 95% ethanol 205 n-hexane 201 methanol 205 isooctane 195 water 190 UV Spectroscopy Aromatic Compounds When the number of fused aromatic rings increases, the l for the primary and secondary bands also increase For heteroaromatic systems spectra become complex with the addition of the n * transition and ring size effects and are unique to each case 259nm 278nm 279nm Infrared Spectra Infrared radiation λ = 2.5 to 17 μm υ = 4000 to 600 cm-1 These frequencies match the frequencies of covalent bond stretching and bending vibrations. Infrared spectroscopy can be used to find out about covalent bonds in molecules. IR is used to tell: 1. what type of bonds are present 2. some structural information Absorption Frequencies of Functional Groups See Appendix B (2 tables) and Table 2 Some characteristic infrared absorption frequencies BOND COMPOUND TYPE FREQUENCY RANGE, cm-1 C-H alkanes 2850-2960 and 1350-1470 alkenes 3020-3080 (m) and RCH=CH2 910-920 and 990-1000 R2C=CH2 880-900 cis-RCH=CHR 675-730 (v) trans-RCH=CHR aromatic rings monosubst. 965-975 3000-3100 (m) and 690-710 and 730-770 ortho-disubst. 735-770 meta-disubst. 690-710 and 750-810 (m) para-disubst. 810-840 (m) alkynes 3300 O-H alcohols or phenols 3200-3640 (b) C=C alkenes 1640-1680 (v) aromatic rings 1500 and 1600 (v) C≡C alkynes 2100-2260 (v) C-O primary alcohols 1050 (b) secondary alcohols 1100 (b) tertiary alcohols 1150 (b) phenols 1230 (b) alkyl ethers 1060-1150 aryl ethers 1200-1275(b) and 1020-1075 (m) all abs. strong unless marked: m, moderate; v, variable; b, broad 1 C5H10O 3400-3200 cm-1: no OH or NH present 3100 cm-1: no peak to suggest unsaturated CH 2900 cm-1: strong peak indicating saturated CH 2200 cm-1: no unsymmetrical triple bonds Structure: IUPAC Name: 3-pentanone 1710 cm-1: strong carbonyl absorbance 1610 cm-1: no absorbance to suggest carbon-carbon double bonds 2 C8H8O 3400-3200 cm-1: no OH or NH present 3100 cm-1: moderate peak suggesting unsaturated CH 2900 cm-1: weak peak indicating possible saturated CH Structure: 2200 cm-1: no unsymmetrical triple bonds IUPAC Name: acetophenone 1690 cm-1: strong carbonyl absorbance 1610 cm-1: weak absorbance bands consistent with carbon-carbon double bonds 3 C7H8O 3400-3200 cm-1: strong peak indicating OH is present 3100 cm-1: weak peak suggesting possible unsaturated CH 2900 cm-1: weak peak indicating possible saturated CH 2200 cm-1: no unsymmetrical triple bonds 1720 cm-1: no carbonyl absorbance 1450-1500 cm-1: moderate absorbance bands consistent with aromatic carbon-carbon double bonds Structure: IUPAC Name: benzyl alcohol Raman Spectroscopy • When radiation passes through a transparent medium, the species present scatter a fraction of the beam in all directions. • In 1928, the Indian physicist C. V. Raman discovered that the visible wavelength of a small fraction of the radiation scattered by certain molecules differs from that of the incident beam and furthermore that the shifts in wavelength depend upon the chemical structure of the molecules responsible for the scattering. Definition of NMR Spectroscopy Nuclear magnetic resonance spectroscopy: commonly referred to as NMR, is a technique which exploits the magnetic properties of certain nuclei to study physical, chemical, and biological properties of matter Compared to mass spectrometry, larger amounts of sample are needed, but nondestructive Energy Differentiation In the presence of an external magnetic field (B0), two spin states exist, +1/2 and -1/2 (For I=1/2). The magnetic moment of the lower energy +1/2 state is aligned with the external field, and that of the higher energy -1/2 spin state is opposed to the external field. Aligned against the applied field Aligned with the applied field g- Values for some nuclei Isotope Net Spin g / MHz T-1 Abundance / % 1H 1/2 42.58 99.98 2H 1 6.54 0.015 3H 1/2 45.41 0.0 31P 1/2 17.25 100.0 23Na 3/2 11.27 100.0 14N 1 3.08 99.63 15N 1/2 4.31 0.37 13C 1/2 10.71 1.108 19F 1/2 40.08 100.0 Schematic NMR Spectrometer Fourier transformation and the NMR spectrum RF Pulse The NMR spectrum Fourier transform The Fourier transform (FT) is a computational method for analyzing the frequencies present in an oscillating signal 1H 1H NMR and 13C NMR Spectrum NMR spectra d ppm Down field 13C NMR spectra d ppm High field Chemical Shift-d When an atom is placed in a magnetic field, its electrons circulate about the direction of the applied magnetic field. This circulation causes a small magnetic field at the nucleus which opposes the externally applied field The magnetic field at the nucleus (the effective field) is therefore generally less than the applied field by a fraction : B = B0(1-s),So u =gB0 (1-s) / 2 Standard for Chemical Shift In NMR spectroscopy, the standard is often tetramethylsilane, Si(CH3)4, abbreviated TMS. Tetramethyl silane (TMS) is used as reference because it is soluble in most organic solvents, is inert, volatile, and has 12 equivalent 1H and 4 equivalent 13C. TMS signal is set to 0