4.13.2012 lecture
advertisement
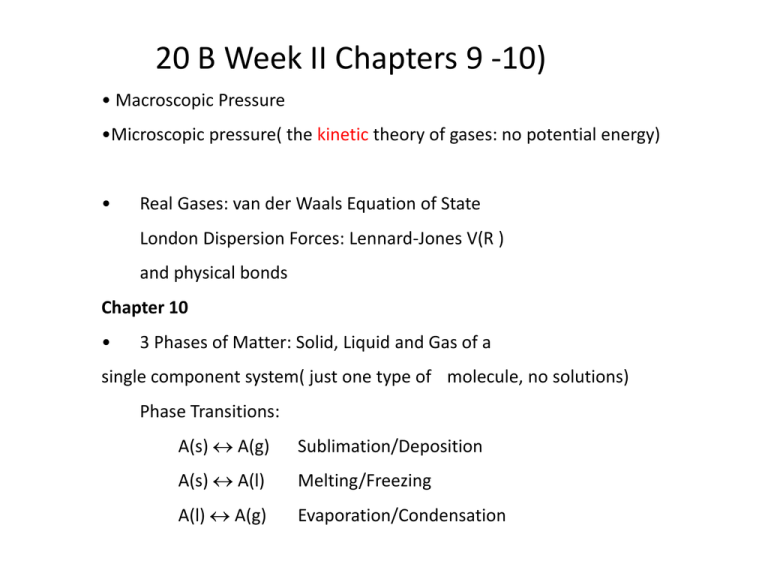
20 B Week II Chapters 9 -10)
• Macroscopic Pressure
•Microscopic pressure( the kinetic theory of gases: no potential energy)
•
Real Gases: van der Waals Equation of State
London Dispersion Forces: Lennard-Jones V(R )
and physical bonds
Chapter 10
•
3 Phases of Matter: Solid, Liquid and Gas of a
single component system( just one type of molecule, no solutions)
Phase Transitions:
A(s) A(g)
Sublimation/Deposition
A(s) A(l)
Melting/Freezing
A(l) A(g)
Evaporation/Condensation
Example:
Volume occupied by a CO2 molecule in the solid compared to
volume associated with CO2 in the gas phase.
The solid. The mass density(r) of solid CO2 (dry ice) r=1.56 g cm-3
1 mole of CO2 molecular weight M=44.01 g mol-1 occupies a molar
volume V= M/r
V= 44.01 g mol-1 /1.56 g cm-3 = 28.3 cm-3 mol-1 1 cm-3 = 10-3 L= mL
Which is approximately the excluded volume per mol-1 = 0.028.3 L mol-1
The Ideal Gas Volume at T=300 K and P=1 atm PV=NkT=nRT
V/n=RT/P= (0.0821 L atm mol-1 K-1)(273 K)/(1 atm) = 22.4 L mol-1
The Real Volume of CO2(g) under these conditions is 22.2 L mol-1
Why is the Real molar volume smaller than the Ideal gas Volume?
Liquid
Solid
>>kT E~PE
Hard Sphere diameter
Gas
<< kT E~KE
Real Gas behavior is more consistent with
the van der Waals Equation of State than PV=nRT
P=[nRT/(V– nb)] – [a(n/V)2] n=N/NA and R=Nak
n= number of moles
b~ NA excluded volume per mole (V-nb) repulsive effect
a represents the attraction between atoms/molecules.
The Equations of State can be determined
from theory or by experimentally fitting P, V, T data!
They are generally more accurate than PV=nRT=NkT
but they are not universal
<V(R )> = 0
2e
2+
R
2e
+2
For R
Very Large
Density N/V is low
Therefore
P=(N/V)kT is low
1 Å = 0.1 nm
Å is an Angstrom
Fig. 9-18, p. 392
Real Gases and Intermolecular Forces
Real Molecular potentials
can be fitted to the form
V(R ) = 4{(R/)12 -(R/)6}
Lennard-Jones Potential
~ hard sphere diameter
well depth or
Dimer Bond
Dissociation
D0=
The London Dispersion
or Induced Dipole Induced Dipole forces
Weakest of the Physical Bonds but it is always present!
Which of these atoms have the
strongest physical bond?
Which of the diatomic molecules
have the strongest physical bond?
Why is CH4 on this list?
Bond dipoles
(kT/ ) ratio predicts deviations from Idea gas behavior.
Since <PE> ~ 0 for real gases
If kT>> which forces are dominant?
Repulsive forces dominate and P>NkT/V for real gases
If kT<< which forces are dominant
Attractive forces dominate and P<NkT/V for real gases
Bond dipoles
(kT/ ) ratio predicts deviations from Idea gas behavior.
Since <PE> ~ 0 for real gases
If kT>> which forces are dominant?
Repulsive forces dominate and P>NkT/V for real gases
If kT<< which forces are dominant
Attractive forces dominate and P<NkT/V for real gases
H2O P-T Phase Diagram
PE
PE+KE
KE
Liquid
Solid
Hard Sphere diameter
Temperature
Gas
<V(R )> = 0
2e
2+
R
2e
+2
For R
Very Large
Density N/V is low
Therefore
P=(N/V)kT is low
Fig. 9-18, p. 392
Real Gases and Intermolecular Forces
Lennard-Jones Potential
V(R ) = 4{(R/)12 -(R/)6}
kT >>
Total Energy
E=KE + V(R)~ KE
Ar+ Ar
/He +
He
well depth is
proportional Ze (or
Mass) but it’s the # of
electrons that control
the well depth
Real Gases and Intermolecular Forces
Lennard-Jones Potential
V(R ) = 4{(R/)12 -(R/)6}
kT <<
well depth
(kT/ ) ratio controls deviations away from Idea gas behavior.
kT>> repulsive forces dominate and P>NkT/V
kT<< attrative forces dominate and P<NkT/V
The effects of the intermolecular force, derived
the potential energy, is seen experimentally through the
Compressibility Factor Z=PV/NkT
Z=PV/NkT>1 when repulsive forces dominate
Z=PV/NkT<1 when attractive forces dominate
Z=PV/NkT=1 when <V(R )>=0 as for the case of an Ideal Gas.
Real Gas behavior is more consistent with
the van der Waals Equation of State than PV=nRT
P=[nRT/(V– nb)] – [a(n/V)2] n=N/NA and R=NAk
b~ NA excluded volume per mole (V-nb) repulsive effect
a represents the attraction between atoms/molecules.
The Equations of State can be determined
from theory or by experimentally fitting P, V, T data!
They are generally more accurate than PV=nRT=NkT
but they are not universal
(kT/ ) ratio controls deviations away from Idea gas behavior.
kT>> repulsive forces dominate and P>NkT/V
kT<< attrative forces dominate and P<NkT/V
The effects of the intermolecular force,
via the potential energy, is seen experimentally through the
Compressibility Factor Z=PV/NkT
Z=PV/NkT>1 when repulsive forces dominate
Z=PV/NkT<1 when attractive forces dominate
Z=PV/NkT=1 when <V(R )>=0 as for the case of an Ideal Gas.
Excluded Volume: (V-nb)~(V - nNA) ~ (V – N )
and
Two Body Attraction: a(n/V)2
The Compressibility factor Z can be written in terms of the van der
Waals Equation of State
Z=PV/nRT= V/{(V-nb) – (a/RT)(n/V)2}
Z= V/{(V-nb) – (a/RT)(n/V)2}=1/{[1-b(n/V)] – (a/RT)(n/V)2}
Repulsion
Z>1
Attraction
Z<1
When a and b are zero, Z = 1 Since PV=RT n=1
Electro-negativity of atoms
In a molecule the more Electronegative atom in a bond will
transfer electron density from the less Electronegative atom
This forms dipole along a bond
e
e
Re
Dipole moment =eRe
A measure of the charge separation along the bond
e
Dipole-Dipole interaction
∂ partial on an atom
Re HCl bond length
e
Dipole moment =eRe
Measure of the charge separation
Real Dimer Structure
Not the Real Dimer Structure
Notice the difference between polar molecules
(dipole moment ≠0)
and non-polar molecules (no net dipole moment =0)
CO2 and CH4
Dipole-Dipole
Hydrogen Bonding due lone
pairs on the O and N atoms
e
e
Dipole moment =eRe
The Potential Energy of Chemical Bonds
Versus Physical Bonds
Physical Bonds
Chemical Bonds