The Heat Capacity of a Solid
advertisement

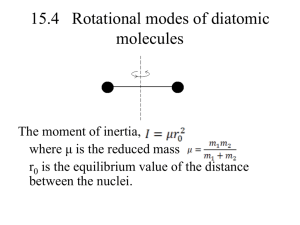
15.5 Electronic Excitation The electronic partition function is where g0 and g1, are, respectively, the degeneracies of the ground state and the first excited state. E1 is the energy separation of the two lowest states. Introducing For most gases, the higher electronic states are not excited (θe ~ 120, 000k for hydrogen). therefore, ln Z U NkT 0 T V 2 U CV 0 T V At practical temperature, electronic excitation makes no contribution to the internal energy or heat capacity! 15.6 The total heat capacity For a diatomic molecule system Since Discussing the relationship of T and Cv (p. 288-289) Heat capacity for diatomic molecules • Example I (problem 15.7) Consider a diatomic gas near room temperature. Show that the entropy is 3 7 2mk 2 T 5 2 S Nk ln 2 , 2 h 2 rot • Solution: For diatomic molecules S Strans S vib S rot S excit At room temperature S excit 0 1 1 Nk e T T 2 e 1 Nk ln S vib 1 e T T 0 (does not contribute!) • For translational motion, the molecules are treated as non-distinguishable assemblies 3 U NkT 2 three degrees of freedom 3 2mkT 2 Z V 2 h U S tr Nk ln Z ln N 1 T 3 5 V 2mkT 2 S tr Nk Nk ln 2 2 N h • For rotational motion (they are distinguishable in terms of kinetic energy) U NkT S 2 rot 1 due to homonuclea r molecule 2 for distinguis U Nk ln Z T Nk Nk ln T Z hable assembly T 2 rot 5 V S system Nk Nk ln 2 N 2mkT 3 2 T Nk Nk ln h 2 2 rot 5 3/ 2 7 V 2mk T 2 S system Nk Nk ln h 2 2 rot 2 N • Example II (problem 15-8) For a kilomole of nitrogen (N2) at standard temperature and pressure, compute (a) the internal energy U; (b) the Helmholtz function F; and (c) the entropy S. • Solution: calculate the characteristic temperature first! vib 3352k and r 2.9k for N 2 U U trans U vib U rot 1 3 1 NkT U NkT Nk vib 2 2 e T 1 because r T U 1 5 1 NkT Nk vib 3352 2 2 e 298 1 1 3352 Nk 2.5 298 3352 11.25 2 e 1 3352 Nk 2272 11.25 e 1 1.0kmol 8.314 103 J kmol1 k 1 2272 1.89 107 J F NkT ln Z ln N 1 non distinguis hable particles F NkT ln Z Distinguis hable particles such as vib & rotation Ft NkT ln Z t ln N 1 2mkT Zt V 2 h 3 2 2 4.65 10 1.38110 22.4m 2 68 6 . 626 10 26 3 22.427.3 10 22.4 273.86 10 19 3 2 20 3 23 Jk 298 1 3 2 2 3 2 3.195 1033 3.195 1033 6 Ft NkT ln 1 NkT ln 5 . 15 10 1 26 6.02 10 NkT 16.4 Fvib NkT ln Z vib Z vib e 3352 1 e 2298 3352 298 e 5.62 1 e 11.24 1 ln Z vib 5.62 ln 1 11.24 5.62 e T Frot NkT ln Z rot Z rot for T rot 2 rot 298 102.72 ln Z rot 4.63 2 * 2.9 Frot 4.63 NkT Z rot F Frot Fvib Ft 4.63 NkT 5.62 NkT 16.4 NkT 15.41NkT 15.41 6.02 10 26 1.38 10 23 298 3.817 107 J Chapter 16: The Heat Capacity of a Solid 16.1 Introduction 1. This is another example that classical kinetic theory cannot provide answers that agree with experimental observations. 2. Dulong and Petit observed in 1819 that the specific heat capacity at constant volume of all elementary solids is approximately 2.49*104 J .kilomole-1 K-1 i.e. 3R. 3. Dulong and Petit’s result can be explained by the principle of equipartition of energy via treating every atom of the solid as a linear oscillator with six degrees of freedom. 4. Extensive studies show that the specific heat capacity of solid varies with temperature, becomes zero as the temperature approaches zero. 5. Specific heat capacities of certain substances such as boron, carbon and silicon are found to be much smaller than 3R at room temperature. 6. The discrepancy between experimental results and theoretical prediction leads to the development of new theories. 16.2 Einstein’s Theory of The Heat Capacity of a Solid • The crystal lattice structure of a solid comprising N atoms can be treated as an assembly of 3N distinguishable onedimensional oscillators! • The assumption is based on that each atom is free to move in three dimensions! From chapter 15: the internal energy for N linear oscillators is U= Nkθ(1/2 + 1/(eθ/T -1)) with θ = hv/k The internal energy of a solid is thus 1 U 3Nk E ( 2 1 E eT ) 1 Here θ is the Einstein temperature and is denoted by θE. The heat capacity: 3Nk E 3Nk E ( E ) 2 T U e 1 Cv T T v Case 1: when T >> θE This result is the same as Dulong & Petit’s Case 2: T << θE As discussed earlier, the increase of powered by the increase of As a result, when is out If an element has a large θE , the ratio will be large even for temperatures well above absolute zero When is large, is small Since hv E k A large θE value means a big On the other hand 1 k v 2 u To achieve a large , we need a large k or a small u (reduced mass), which corresponds to a light element or elements that produce very hard crystals. • The essential behavior of the specific heat capacity of solid is incorporated in the ratio of θE/T. • For example, the heat capacity of diamond approaches 3Nk only at extremely high temperatures as θE = 1450 k for diamond. • Different elements at different temperatures will poses the same specific heat capacity if the ratio θE/T is the same. • Careful measurements of heat capacity show that Einstein’s model gives results which are slightly below experimental values in the transition range of