phys-4420 thermodynamics & statistical mechanics spring 2006
advertisement
PHYS-4420 THERMODYNAMICS & STATISTICAL MECHANICS
Quiz 2
SPRING 2006
Friday, April 21, 2006
NAME: __________ANSWERS____________________
To receive credit for a problem, you must show your work, or explain how you arrived at your
answer.
Credit Grade
1
15%
2
20%
3
50%
4
15%
Total 100%
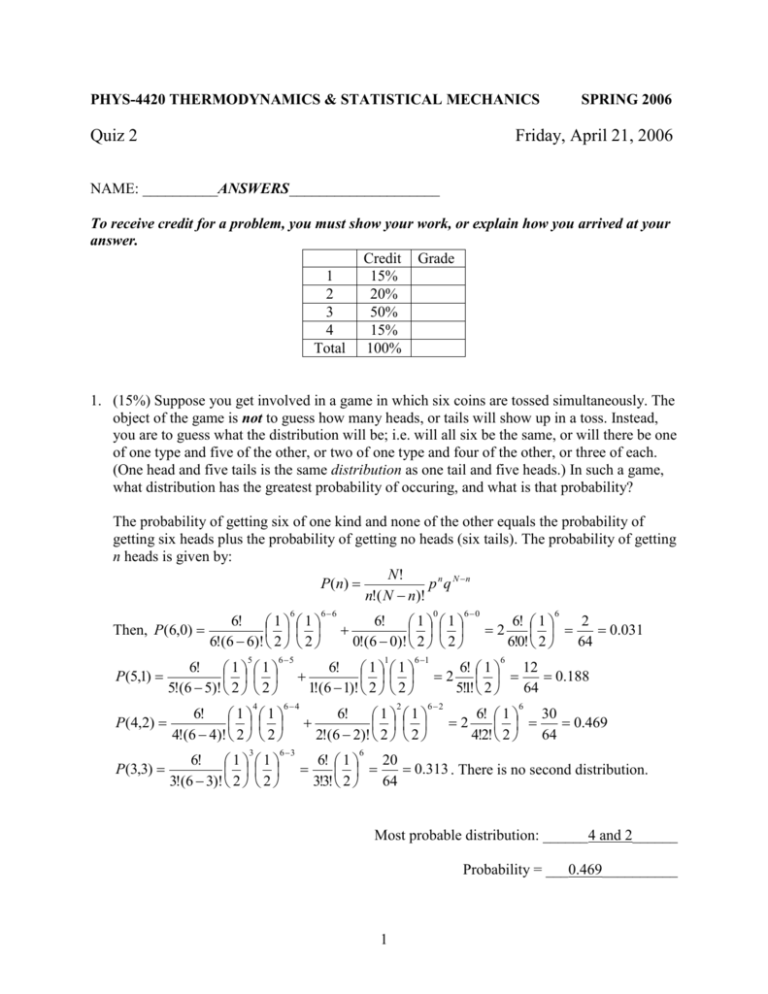
1. (15%) Suppose you get involved in a game in which six coins are tossed simultaneously. The
object of the game is not to guess how many heads, or tails will show up in a toss. Instead,
you are to guess what the distribution will be; i.e. will all six be the same, or will there be one
of one type and five of the other, or two of one type and four of the other, or three of each.
(One head and five tails is the same distribution as one tail and five heads.) In such a game,
what distribution has the greatest probability of occuring, and what is that probability?
The probability of getting six of one kind and none of the other equals the probability of
getting six heads plus the probability of getting no heads (six tails). The probability of getting
n heads is given by:
N!
P ( n)
p n q N n
n!( N n)!
6
6!
1 1
Then, P(6,0)
6!(6 6)! 2 2
P(5,1)
6! 1
5!(6 5)! 2
5
1
2
65
4
3
6! 1 1
P(3,3)
3!(6 3)! 2 2
64
63
0
6!
1 1
0!(6 0)! 2 2
1
6!
1 1
P(4,2)
4!(6 4)! 2 2
66
6! 1
1!(6 1)! 2
1
2
2
6 1
6!
1 1
2!(6 2)! 2 2
60
6
6! 1
2
2
0.031
6!0! 2
64
6
2
62
6! 1
12
0.188
5!1! 2
64
6
6! 1
30
2
0.469
4!2! 2
64
6
6! 1
20
0.313 . There is no second distribution.
3!3! 2
64
Most probable distribution: ______4 and 2______
Probability = ___0.469__________
1
2. (20%) For a certain system, there is only one accessible state and it has energy,
V
s NkT ln
V0
where V0 is a constant.
a) (10%) What is the partition function for this system?
Z e
s
e
V
NkT ln
V0
e
1
kT
V
NkT ln
V0
s
e
V
N ln
V0
e
V
ln
V0
N
V
V0
N
V
Z
V0
N
b) (10%) Use the result of part a) to find the average pressure for this system as a function
of temperature and volume.
N
2
ln Z
V
ln NkT
N ln V N ln V0 T N kT
P NkT
NkT
V
V V0
V
V
T
N 2 kT
P
V
or, F NkT (ln Z ln N 1) NkT ( N ln V N ln V ln N 10 ) , and
P
F N 2 kT
, as above.
V
V
2
3. (50%) Consider a crystal with N atoms. Suppose that the atoms can be excited out of their
crystal lattice positions (of which there are N) into interstitial sites (of which there are also
N). The energy of this excitation is for each atom that is excited. Ignore lattice vibrations
and any other possible degree of freedom. The interstitials do not interact with each other,
however, two atoms cannot both be on the same interstitial site. The crystal is in contact with
a heat reservoir which maintains it at a constant temperature T and provides energy to create
the interstitials.
a) (12%) When n atoms are excited to interstitial positions, how many microstates w are
available to the system?
N!
N!
N!
w wl wi
n!( N n)! n!( N n)! n!( N n)!
2
N!
w
n!( N n)!
2
b) (13%) Use your answer to a) to calculate the entropy of the system. Your result should
contain factorials. Apply Stirling’s approximation to your result, since N and n will be
large enough to justify its use.
2
N!
2k[ln N! ln n! ln( N n)!]
S k ln w k ln
n!( N n)!
S 2k[ln N! ln n! ln( N n)!] 2k[ N ln N N n ln n n ( N n) ln( N n) N n)]
S 2k[ N ln N n ln n ( N n) ln( N n)]
S 2k[ N ln N n ln n ( N n) ln( N n)]
c) (10%)Since the crystal is able to exchange energy with the reservoir, it will be at
equilibrium when the Helmholtz function (F = U – TS) is a minimum. Find the
Helmholtz function for this crystal, considering only the energy and entropy associated
with the interstitials.
F U TS n T 2k[ N ln N n ln n ( N n) ln( N n)]
F n 2kT[ N ln N n ln n ( N n) ln( N n)]
3
d) (15%) Use the Helmholtz function just calculated to find an expression for the number of
interstitials that can be expected at temperature T.
Find where
F
0.
n
F
{n 2kT[ N ln N n ln n ( N n) ln( N n)]} 0
n n
n
N n
2kT ln n ln( N n)
0
n
N n
N n
2kT ln n ln( N n) 2kT ln
n
N n
2kT ln
2kT
n
N n
e 2 kT
so ne 2 kT N n and n e 2 kT 1 N
n
n
N
e 2 kT 1
When n << N, which is normal, e
2 kT
1 , so n Ne
2 kT
4. (15%) An astronomer observes the light from a distant gas cloud in space. A wavelength
analysis of the light reveals two sharp lines which are characteristic of two energy transitions
in a particular molecule. The lower energy line (2.1 × 10-3 eV) corresponds to the energy for
a transition between the first excited state and the ground state while the higher energy line
(3.2 × 10-3 eV) corresponds to the transition between the second excited state and the ground
state. (These energies are determined in the rest frame of the cloud; all relativistic
corrections due to motion of the cloud with respect to earth have already been included. This
is mentioned for those of you that would think of this complication. Please, do not worry
about it here.) Based on the measured intensities of the two lines, the astronomer determines
that there are 5 times more molecules in the first excited state than there are in the second
excited state. Compute the temperature of the gas cloud based on this information.
fi N
T
e
i
kT
Z
, so
1
f1 e kT
e
f2
e kT
2
2 1
kT
f
1
. Then, ln 1 2 1 , so T 2
kT
f
f2
k ln 1
f2
3.2 103 eV 2.1 103 eV
(8.62 105 eV/K) ln 5
T = 7.9 K
4