e- arrangement - Swiftchem.org
advertisement

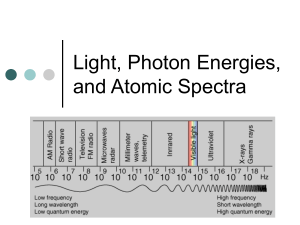
Electron Arrangement * 2.3 Electron arrangement Assessment statement 2.3.1 Describe the electromagnetic spectrum. Teacher’s notes Students should be able to identify the ultraviolet, visible and infrared regions, and to describe the variation in wavelength, frequency and energy across the spectrum. 2.3.2 Distinguish between a continuous spectrum and a line spectrum. Students should be able to draw an energy level 2.3.3 Explain how the lines in the emission spectrum of hydrogen are related to electron diagram, show transitions between different energy levels and recognize that the lines in a energy levels. line spectrum are directly related to these differences. An understanding of convergence is expected. Series should be considered in the ultraviolet, visible and infrared regions of the spectrum. Calculations, knowledge of quantum numbers and historical references will not be assessed. 2.3.4 Deduce the electron arrangement for atoms and ions up to Z = 20. For example, 2.8.7 or 2,8,7 for Z = 17. Electron Arrangement 2.3.1 * Assessment statement Teacher’s notes Describe the electromagnetic spectrum. Students should be able to identify the ultraviolet, visible and infrared regions, and to describe the variation in wavelength, frequency and energy across the spectrum. • The study of the emission of light by atoms and ions is the most effective technique for determining the structure of atoms. Blackbody Radiation: (Heating metal to glow RED Or even WHITE) Max Planck, 1900: Energy, like matter, is discontinuous. E = h Prentice-Hall © 2002 General Chemistry: Chapter 9 Slide 4 of 50 The Nature of Energy • Einstein used this assumption to explain the photoelectric effect. • He concluded that energy is proportional to frequency: E = hf where h is Planck’s constant, 6.63 10−34 J-s. * * The Photoelectric Effect • Light striking the surface of certain metals causes ejection of electrons. • Depends on WAVELENGTH, not intensity Prentice-Hall © 2002 General Chemistry: Chapter 9 Slide 6 of 50 * The Nature of Energy • Therefore, if the wavelength of light is known, the energy of that light (one photon, a packet) can be calculated, : E = hf (packet = quanta) * Wavelength • The color of light is sometimes defined by its wavelength (λ)(lambda) rather than its frequency ()(nu). • The two are related by the equation c = λ • All electromagnetic radiation travels at the same velocity: the speed of light (c) =2.997925 x 108 m s-1 (3.00 108 ms-1 ) (meters per second) • Frequency () (nu) in Hertz—Hz or s-1. • Wavelength (λ) (lambda) in meters—m. • cm m nm Angstrom (10-2 m) (10-6 m) (10-9 m) (10-10 m) Equations: c = λ λ = c/ (c = lambda nu) = c/λ pm (10-12 m) Wavelength • The greater the velocity (the higher the frequency), the shorter the wavelength. Low High * Wavelength Prentice-Hall © 2002 General Chemistry: Chapter 9 Slide 11 of 50 * ROYGBIV Red Orange Yellow 700 nm 450 nm Green Blue Indigo Violet Prentice-Hall © 2002 General Chemistry: Chapter 9 Slide 12 of 50 Continuous vs Line Spectrums Assessment statement 2.3.2 * Teacher’s notes Distinguish between a continuous spectrum and a line spectrum. • Electrons surround the nucleus in specific orbitals or energy levels. • When an element is excited, it will often emit a light of a characteristic color. • Gases can be excited by passing an electrical discharge at low pressure. • When this excited gas or excited elements light is viewed through a spectroscope a continuous spectrum is not observed, but a series of very bright lines of specific colors with black spaces in-between instead. • • This is known as a line spectrum. Continuous vs Line Spectrums * In a continuous spectrum, all wavelengths of visible light are represented (like a rainbow) as is observed with “white” light. Continuous vs Line Spectrums In a line spectrum, only specific wavelengths are represented. It comprises very bright lines of specific colors with black space in between. This is from an emission (energized samples “glowing”.) Line spectra are unique for every element and are used to identify atoms (much like fingerprints are used to identify people). * Continuous vs Line Spectrums In a Absorption spectrum, only specific wavelengths are missing from a continuous spectrum. It comprises colors with black lines. This is an absorption (a source of white light is being filtered by a sample) * Continuous vs Absorption/Emission * * Atomic Emission Spectra • When an atom is excited, its electrons gain energy and move to a higher energy level. • In order to return to the lower energy levels, the electron must lose energy. • It does this by giving out light. Atomic Line Spectra 1. An electron in the atom gains (absorbs) energy from heating. 2. Electron jumps up an energy level. 3. Electron is now unstable (unwelcome) in this level and is “kicked out”. 4. When the electron loses the energy and come back to the original level, light is emitted. * Energy Levels 2.3.3 * Assessment statement Teacher’s notes Explain how the lines in the emission spectrum of hydrogen are related to electron energy levels. Students should be able to draw an energy level diagram, show transitions between different energy levels and recognize that the lines in a line spectrum are directly related to these differences. An understanding of convergence is expected. Series should be considered in the ultraviolet, visible and infrared regions of the spectrum. Calculations, knowledge of quantum numbers and historical references will not be assessed. Energy Levels • Because there are only certain allowed energy levels in the atom, there are a limited number of amounts of energy that an electron can lose. • The energy levels of atoms are not evenly spaced, like the rungs of a ladder, but that the higher the energy level, the smaller the difference in energy between successive energy levels becomes. • This means that only certain frequencies can emit light and this is why we get the line spectrum. • This means that the lines of a spectrum will converge (get closer together with increasing energy). • The limit of this convergence indicates the energy required to completely remove the electron from the atom (to ionize it) and so may be used to determine the ionization energy. * Energy Levels The Atomic Emission Spectrum of Hydrogen • The emission spectrum of hydrogen is the simplest emission spectrum because there is no electron – electron repulsion which will cause the principle energy levels to split into different sub levels. • When a voltage is applied across hydrogen gas, electromagnetic radiation is emitted. • This is not uniform, but concentrated into bright lines, indicating the existence of only certain allowed electron energy levels. • The study of this spectra allowed for the electron structure of hydrogen to be deduced. * The Atomic Emission Spectrum of Hydrogen The Atomic Emission Spectrum of Hydrogen • The spectrum is divided into three distinct series which occur in different spectral regions (visible, IR, UV). • Each series corresponds to transitions in which the electron falls to a particular energy level. • The reason why they occur in different spectral regions is that as the energy level increases, they converge (they get closer together in energy). • This means that all transitions to the n=1 level include the large n=1 to n=2 energy difference and so they are all high energy transitions found in the UV region. • For similar reasons, all transitions to the n=2 level are in the visible region. • All transitions to the n=3 level are in the infrared region. * * Electron Structure and the Periodic Table 2.3.4 Assessment statement Teacher’s notes Deduce the electron arrangement for atoms and ions up to Z = 20. For example, 2.8.7 or 2,8,7 for Z = 17. • The most stable energy levels, or shells, are those closest to the nucleus and these are filled before electrons start to fill the higher levels. • There is a maximum number of electrons that each energy level can hold. – The first can hold 2 – The second can hold up to 8 – Beyond this the situation gets more complex Electronic Structure * • The number of electrons in each orbital is known as the electronic structure of the atom. • Example Aluminum – Aluminum has 13 electrons • 2,8,3 is the electronic structure. • 2 in the first, 8 in the second and 3 in the third • It is the electrons in the outermost (valence) shell that determine the physical and chemical properties of the element. • Elements with three or less electrons in the valence shell are metals (except boron). • The electronic structure is closely related to the position on the periodic table. • The period from the periodic table gives us the number of energy levels that contain electrons. • The group on the period table gives us the number of electrons in the valence shell. Electronic Structure of the Periodic Table (up to Z=20) * Electronic Structure of the Periodic Table * Electronic Structure of the Periodic Table *