Option B Quantum and Nuclear Physics
advertisement

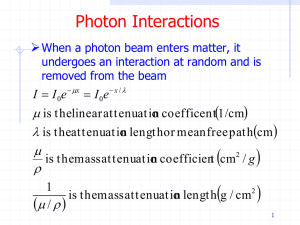
OPTION B QUANTUM AND NUCLEAR PHYSICS Also know as Topic:13 These notes were typed in association with Physics for use with the IB Diploma Programme by Michael Dickinson For further reading and explanation see: Physics, Tsokos (purple): Ch 6.4 Physics, Giancoli (mountain): Ch 27 13.1.1 – Describe the photoelectric effect 13.1.2 – Describe the concept of the photon, and use it to explain the photoelectric effect. 13.1.3 – Describe and explain an experiment to test the Einstein model. • Frist off lets get a quick summary of everything. Try this link. http://www.youtube.com/watch?v=WaZdgrwm2dw&list=PL80C5AF53 6A5A90DF&index=1 • So that’s were we are going. 13.1.1 – Describe the photoelectric effect 13.1.2 – Describe the concept of the photon, and use it to explain the photoelectric effect. 13.1.3 – Describe and explain an experiment to test the Einstein model. • Things and get a little tricky so hang on and review often. • Photoelectric Effect - When light shines on a clean metal surface, electrons are emitted from the surface. • Demo http://www.youtube.com/watch?v=WO38qVDGgqw&list=PL80C5AF5 36A5A90DF • Explination http://www.youtube.com/watch?v=N7BywkIretM&list=PL80C5AF536 A5A90DF Key Point • The light has to have a sufficiently high frequency, called the cut off or threshold frequency, f0. 13.1.1 – Describe the photoelectric effect 13.1.2 – Describe the concept of the photon, and use it to explain the photoelectric effect. 13.1.3 – Describe and explain an experiment to test the Einstein model. • Cathode – negatively charged electrode, electrons flow away from this • Anode – positively charged electrode, electrons flow toward this Common Demo • Occurs in a vacuum tube 13.1.1 – Describe the photoelectric effect 13.1.2 – Describe the concept of the photon, and use it to explain the photoelectric effect. 13.1.3 – Describe and explain an experiment to test the Einstein model. • Millikan’s Experiment • Applies a variable potential difference across the electrodes. This produces an opposing electric field to the movement of the ejected electrons. • The reverse potential, or stopping Potential, Vs, is adjusted until the ammeter is zero. • The stopping potential is the max kinetic energy of the ejected electrons. 13.1.1 – Describe the photoelectric effect 13.1.2 – Describe the concept of the photon, and use it to explain the photoelectric effect. 13.1.3 – Describe and explain an experiment to test the Einstein model. • Millikan’s Experiment • EK(max) = Eelec • ½ mv2 = eVs • Where m is the mass of and electron e is the charge magnitude and Vs is the stopping voltage. 13.1.1 – Describe the photoelectric effect 13.1.2 – Describe the concept of the photon, and use it to explain the photoelectric effect. 13.1.3 – Describe and explain an experiment to test the Einstein model. • Millikan’s Experiment • Applies a variable potential difference across the electrodes. This produces an opposing electric field to the movement of the ejected electrons. • The reverse potential, or stopping Potential, Vs, is adjusted until the ammeter is zero. • The stopping potential is the max kinetic energy of the ejected electrons. 13.1.1 – Describe the photoelectric effect 13.1.2 – Describe the concept of the photon, and use it to explain the photoelectric effect. 13.1.3 – Describe and explain an experiment to test the Einstein model. Frequency vs. max kinetic energy graph • Increase the frequency of the light shining on the metal, there is an increase in kinetic energy of the ejected electrons. • The intensity of the incident light is proportional to the number of electron emitted. But also an increase in intensity didn’t change the energy of the electrons emitted. 13.1.1 – Describe the photoelectric effect 13.1.2 – Describe the concept of the photon, and use it to explain the photoelectric effect. 13.1.3 – Describe and explain an experiment to test the Einstein model. Millikan’s Experiment • Why did the metal not emit electrons immediately, but did so after a certain frequency. • The light has to have a sufficiently high frequency, called the cut off or threshold frequency, f0. 13.1.1 – Describe the photoelectric effect 13.1.2 – Describe the concept of the photon, and use it to explain the photoelectric effect. 13.1.3 – Describe and explain an experiment to test the Einstein model. • Einstein continued Max Planck’s work and developed the particle theory. • Planck observed that energy released from vibrating molecules were always in packets called quanta of energy. • Einstein said that light originates from a vibrating source then light energy could be quantized particles called photons. • Each with an energy of E = hf Where E is energy, h is Planck’s constant, f is frequency. • With this theory everything started to fall in place. • EK(max) = hf = eVs • Increasing the intensity of light at constant frequency means a greater quantity of electrons would be ejected, but does not increase the energy of each photon and so does not increase the max kinetic energy of the ejected electron. 13.1.1 – Describe the photoelectric effect 13.1.2 – Describe the concept of the photon, and use it to explain the photoelectric effect. 13.1.3 – Describe and explain an experiment to test the Einstein model. • At low frequencies the photon energy is low and electrons are not emitted. • Work Function Φ – the minimum amount of energy of photons incident on a surface required to cause photoelectric emission. • Φ = hf0 • From, E = hf we can say… IB Equations • hf = Φ + EK(max) • hf = hf0 + eV 13.1.1 – Describe the photoelectric effect 13.1.2 – Describe the concept of the photon, and use it to explain the photoelectric effect. 13.1.3 – Describe and explain an experiment to test the Einstein model. • All this can be arranged in y = mx + b form… • eVs = hf – hf0 • y is eVs or EK(max) • m is planck’s constnat or h • b is hf0 or Φ IB Definition • h – planck’s constant • Is 6.63 x 10-34 Js