x y - Deans Community High School
advertisement

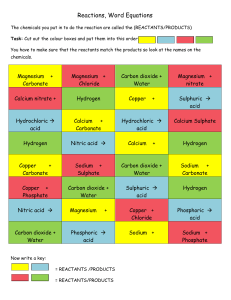
Name: ________________________ Teacher: ___________________ Homework 1– Chemical Reactions 1. Write down three examples of everyday chemical reactions. 2. Write word equations for the following reactions: (a) Hydrogen joins up with oxygen to form water. (b) The reaction of sulphuric acid with calcium carbonate produces calcium sulphate, carbon dioxide and water. (c) Silver oxide breaks up when heated to form its elements. (d) Ammonium sulphate is produced by the reactions of ammonia with sulphuric acid. 3. If a substance effervesces (fizzes), is there a chemical reaction taking place? Explain your answer. 4. The first 20 elements (hydrogen to calcium) in the periodic table were all discovered before the twentieth century began. Use the data booklet (page 8) to help you answer the following questions about these elements. (a) Which two elements were known in prehistoric times? (b) Which elements were discovered in the 18th century? (c) Name three elements from your periodic table which are named after famous scientists. 5. Name two elements which are: a b c d e f g metallic non-metallic solid at room temperature liquid at room temperature gas at room temperature naturally occurring made by scientists Name: ________________________ Teacher: ___________________ 6. The elements are arranged in the Periodic Table a What is meant by a group? b What is meant by a period? c Give an example of a physical similarity of elements in group 1. d Give an example of a chemical similarity of elements in group 1. 7. Divide the following list of substances into elements and compounds. carbon, petrol, alcohol, zinc, oxygen, sugar, sodium chloride, gold, copper sulphate, nitrogen, aluminium, silver oxide 8. Explain what is meant by (a) an element (b) a compound (c) a mixture (d) a solute (e) a solvent (f) a solution 9. Name the compound which is formed when each of the following pairs of elements join up. (i) magnesium and chlorine (ii) lead and sulphur (iii) sodium and oxygen (iv) hydrogen and iodine 10. Name two possible compounds containing potassium, sulphur and oxygen. 11. (a) (b) 12. What elements are present in: (a) (b) 13. How many elements are there in compounds whose names end in “ide”. If a compounds name ends in “ite” or “ate”, what does this tell you about the number of elements in the compound and which element is always present? sulphur oxide potassium sulphate (c) (d) phosphorus chloride lithium sulphite Crude oil is a mixture of compounds. Explain clearly what is meant by this statement Name: ________________________ Teacher: ___________________ Homework 2 – Speed of Reactions 1. List the following in order of rate of reaction, fastest first: Milk turning sour A motor car rusting Frying an egg Striking a match 2. The grid shows factors that can affect the speed of a chemical reaction. A B increase in particle size D C increase in concentration E decrease in particle size increase in temperature F decrease in concentration decrease in temperature Identify the factor responsible for the change in speed of reaction in each of the following examples. (a) Seawater corrodes iron jetties near the Equator more quickly than in northern Scandinavia. (b) Magnesium ribbon burns more slowly than magnesium powder. (c) Two scoops of biological detergent in a washing machine remove stains from clothing more quickly than using one scoop. 3. The speed of a reaction depends on the reaction conditions. Describe how each of the following affects the speed of a reaction. a) particle size of the reactants b) concentration of reactants c) temperature of reactants d) using a catalyst 4. Explain each of the following a) A log burns faster if it is chopped into small sticks b) Food is preserved longer when stored in a fridge c) Plants grow faster in a greenhouse than in the open air 5. Three experiments were set up as shown. Each experiment is Name: ________________________ Teacher: ___________________ carried out at room temperature and the mass of magnesium is the same in each case. (a) Explain any difference that would be observed between (i) A and B (ii) A and C A was (b) Explain any difference that would be observed if experiment repeated at 50oC Name: ________________________ Teacher: ___________________ Homework 3 – Speed of Reactions Interpreting Graphs 1. The graph shows the volume of carbon dioxide produced in the reaction of (i) calcium carbonate powder (ii) calcium carbonate lumps with excess dilute hydrochloric acid Volume of carbon dioxide / cm3 x y Time / s a) b) 2. Which graph, X or Y, shows the results of using calcium carbonate powder? State three variables that must be kept the same when comparing the reactions 5g of magnesium ribbon was added to excess sulphuric acid and the Volume of hydrogen / cm3 Time / s volume of hydrogen gas evolved was measured. A graph of the result is shown below:- Name: ________________________ a) b) Teacher: ___________________ Copy the graph and draw a line to show how the volume of hydrogen produced would compare if 5g of magnesium powder was added to the acid. Draw another line of the graph showing the volume of hydrogen produced if 2.5g of magnesium ribbon was added.