2013 lecture 2
advertisement
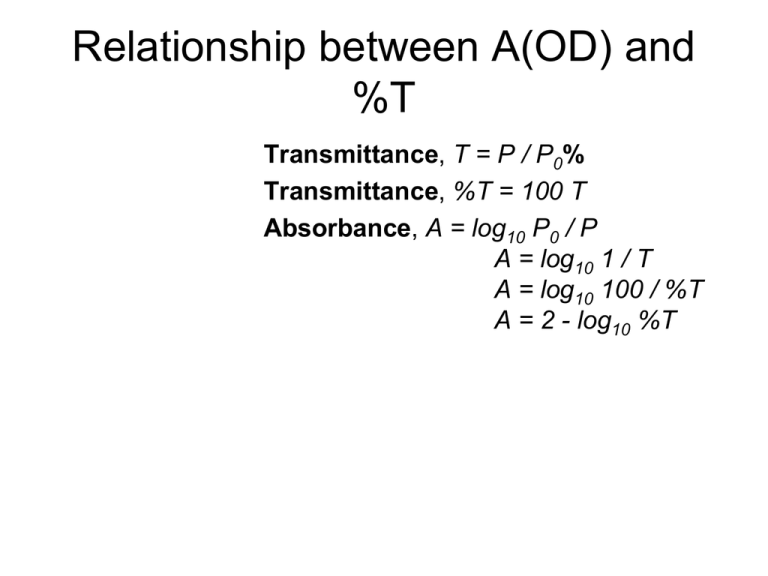
Relationship between A(OD) and %T Transmittance, T = P / P0% Transmittance, %T = 100 T Absorbance, A = log10 P0 / P A = log10 1 / T A = log10 100 / %T A = 2 - log10 %T Beer Lamert’s Law Reflection Light scattering reflection scattering For Solution: Scattering ~ 1/4 UV-Vis Spectrum of Milk Prism Diffraction grating Spectrophotometer types -Single beam -Dual beam -Diode array Single Beam - Spectrophotometer Dual Beam - Spectrophotometer Dual Beam – Single Detector Diode Array - Spectrophotometer NanoDrop Bradford Assay Substrate (S) and enzyme (E) combine to form the enzyme/substrate complex (ES). The complex then dissociates to yield enzyme (E) plus product (P). ELISA Enzyme-Linked Immunosorbent Assay LDH Cytotoxicity Assay Endpoint vs Kinetic Endpoint vs Kinetic Buffer Dilution • V1 x C1 = ? V2 x C2 Example: Need to make 1 L of 1mg/mL solution given 100mg/mL stock Example 2: Need to add component from 5.2x stock to 200mL of sample Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. George Gabriel Stokes named the phenomenon fluorescence in 1852. The name was derived from the mineral fluorite (calcium difluoride) Molecular Orbital Factors that influence on Fluorescence pH Solid state or Solution state Solvent Energy Absorbance Fluorescence Vibrational and rotational relaxation The excitation and emission spectra of a fluorophore and the correlation between the excitation amplitude and the emission intensity. General diagram of the excitation and emission spectra for a fluorophore (left). The intensity of the emitted light (Em1 and Em2) is directly proportional to the energy required to excite a fluorophore at any excitation wavelength (Ex1 and Ex2, respectively; right). The Stokes shift of the excitation and emission spectra of a fluorophore. Fluorophores with greater Stokes shifts (left) show clear distinction between excitation and emission light in a sample, while fluorophores with smaller Stokes shifts (right) exhibit greater background signal because of the smaller difference between excitation and emission wavelengths. scattering Exitation reflection Emission Spectrofluorometer Detector monochromator Emission Excitation scattering Exitation reflection Emission Microscope and Plate Reader Detector Filter Excitation Emission Dichroic Mirror scattering Exitation reflection Emission Optical Path Microplate Reader scattering Exitation reflection Emission Filter and Dichroic Mirror http://www.chroma.com/products/catalog/11000_Series/11000v3 http://www.invitrogen.com/site/us/en/home/support/Research-Tools/Fluorescence-SpectraViewer.html https://www.omegafilters.com/curvo2/index.php