STED-04-24
advertisement

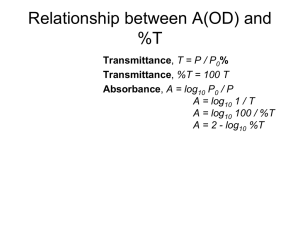
FLUORESCENCE IMAGING I. Fluorescence-imaging with diffraction limited spots The resolution in optical microscopy has been hampered by the smallest spot possible (~/2) that can be achieved by conventional methods. y x Sample region with fluorescent labeled molecules Spatial distribution of the excitation Diffraction-limited beam Spot size Fig.1. Excitation with a diffraction limited beam. The minimum area that can be excited is determined by the ability to create a spot with the minimum size. II. High resolution fluorescence-imaging A key aspect is to find a way to reduce the number of fluorescently labeled molecules that are excited simultaneously. Here we mentioned a couple of methods that have been successfully applied. II.1 Stimulated Emission depletion (STED) In this approach, the effective size of the exciting beam is reduced by quenching (reducing) the fluorescence emission of the fluorophores located in the periphery of the excitation beam with a doughnut-shaped beam. Fig.2 Experimental setup (left) for creating a doughnut shape beam Right) for quenching the fluorophore emission.1 Although the application of a laser to quench the fluorescence may appear counterintuitive, this is indeed what happens (as will be explained below.) As a result, the effective area of excitation is reduced. “The result is akin to sharpening a pencil to draw finer lines. By scanning the ‘sharpened spot over the sample, an image Is built pixel by pixel, with a resolution currently down to 20 nm.” [Ref. F. Pinaud and M. Dahan, 2008] Quenched molecules Fluorescence emission Fig.3 At the focal point, the effective excitation beam is much narrower than lambda.2 A key feature in STED is that, it turns out, the effective quenching process depends non-linearly with the intensity of the quenching beam. SHATTERING the DIFFRACTION LIMIT of LIGHT I. STRATEGIES IA. Volumetric-shaping of the excitation light Most recent superresolution methods rely on the volumetric shaping of the excitation light (through a near-field aperture tip; periodic light gradients generated by interference, as in 4PI.) IB. Exploiting non-linear light-matter interactions More specifically, using a nonlinear relationship between the excitation and the fluorescence emission IB.1 Non-resonant Processes: Two- and multiphoton excitations But they suffer from two main drawbacks. a) Fluorophores that emit in the visible require multiphoton excitation with doubled or tripled wavelength, which results in the doubling or tripling of the extent of the excitation spot (hence spoiling the resolution gained by the non liner process.) b) Multi-photon excitations are higher-order non-resonant processes. Consequently, their absorption cross sections are many orders of magnitude smaller than that of the linear one-photon process, which forces the use of intense short laser pulses for efficient excitation. The latter are phototoxic to the cell because they accelerate radical production, thereby limiting the available observation time before cell damage. IB.2 Resonant processes: Exploit spectroscopic properties of fluorophores to produce nonlinearities of large cross sections. Previously fluorescence has been treated as a linear process. But Hell’s group has been looking for ways to exploit the spectroscopic properties of fuorophores to produce non-linearities. This effort has led to the Stimilated Emission Depletion (STED). From reference: S. Weiss, “Shattering the diffraction limit of light,” PNAS 97, 8747 (2000) II. STIMULATED EMISSION DEPLETION (STED): Exploiting non-lnearities in resonant processes In 1994 Jan Wichmann and Hell published a theoretical paper on STED, outlining a concept to eliminate the resolution-limiting effect of diffraction without eliminating diffraction itself. Reference: Stefan W. Hell and Jan Wichmann, “Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy,” OPTICS LETTERS 19, 780 (1994). STED exploit the selective quenching : a) Excitation process A short pulse 200 fs and exc~ 560 nm excites the fluorophores from S0 into high vibrational states S1 vib. y Spatial distribution of the excitation beam x 0.2 ps pulse Time S1vib S1 Sovib So Fig.1 Spatial (left) and temporal (center) distribution of the excitation beam. The diagram on the right side displays the energy levels of a typical fluorophore. S0 and S1 are the ground and first excited singlet states, respectively. S0vib , and S1 vib are higher vibrational levels of these states. The excitation of the dye takes place from the relaxed state S0 to the states S1 vib. The intensity distribution of the excitation beam in the focal plane of the lens is determined by diffraction effects. 2 I exc (r ) ~ J1 (r ) / r ( Joules per unit area per unit time.) where r ( x 2 y 2 )1/ 2 , and J1 is the first order Bessel function. The spatial extent of I exc (r ) determines the resolution of the microscope. The efficiency of absorption is characterized by the absorption cross section coefficient 01 . 01 ~ in the 10-16 to 10-17 cm2 range If no is the population of the state S0, the temporal population change due to stimulated absorption is given by, dno no 01I exc / dt b) Relaxation processes Vibrational relaxations S1 vib S1 occurs in ~ 1 to 5 ps. Fluorescence by radiative transitions occurs due to transitions S1 Sovib ; with an average fluorescence lifetime fluor ~ 2 n ps. This is three orders of magnitude slower than the vibrational transitions. S1vib S1 ps S1vib S1 S1vib S1 ns So vib So So vib Sovib So So ps The transition from S1 to Sovib can also be induced by stimulated emission, which is of particular interest here. The transitions Svib 1 ! S1 and Svib 0 ! S0 are vibrational relaxations. In the discussion of stimulated emission we can ignore the triplet state. Detailed reviews of dye properties are given by Lakowicz [12] and Sch¨afer [13]. Figure 1 also displays Iexc = 1300 MW/cm2. I exc (r ) / photons per unit are per second, quantifies the probability that an excitation photon arrives at r. Stimulated emission is the basis of laser action and one of the most widely applied physical phenomena. First reports of stimulated emission in organic fluorophores go back to Sorokin and Lankard [5] and to Sch¨afer and coworkers [6], who pioneered the development of the dye laser. The operational requirements in a laser are somewhat different than those for depletion of fluorescence. In a laser, the role of stimulated emission is to strengthen the beam by collecting stimulated photons, whereas in microscopy one is primarily interested in the depletion of the excited state by stimulated emission, irrespective of the population of the excited state. When analyzing a sample containing organic fluorophores, STED exploits a) the depletion of a molecular fluorescent state through stimulated emission, and b) the fact that the product if two point-spread-functions (PSF) is narrower than a single PSF. The first aspect is to it was experimentally demonstrated in 1999. References: Thomas A. Klar and Stefan W. Hell, “Subdiffraction resolution in far-field fluorescence microscopy,” OPTICS LETTERS. 24, 954, 1999. Thomas A. Klar, Stefan Jakobs, Marcus Dyba, Alexander Egner, and Stefan W. Hellt, „Fluorescence microscopy with diffraction resolution barrier roken bty stimulated emission,“ PNAS 97, 8206 (2000). Thomas A. Klar, Egbert Engel, and Stefan W. Hell,“ Breaking Abbe’s diffraction resolution limit in fluorescence microscopy with stimulated emission depletion beams of various shapes,” Phys. Rev. 64, 066613 (2001). 2 Fabien Pinaud and Maxime Dahan, “Zooming Into Live Cells,” Science 320, 187 (2008). 1