UV-Visible Molecular Absorption Spectrometry
advertisement

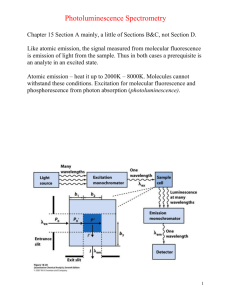
Molecular Fluorescence/Phosphorescence Chapter 15 Section A mainly, a little of Sections B&C, not Section D. Like atomic emission, the signal measured from molecular fluorescence is emission of light from the sample. Thus in both cases a prerequisite is an analyte in an excited state. Atomic emission – heat it up to 2000K – 8000K. Molecules cannot withstand these conditions. Excitation for molecular fluorescence and phosphorescence from photon absorption (photoluminescence). 1 The relative rates of these various processes are important. Process Rate (s) Absorption 10-15 Vibrational Relaxation 10-12 Fluorescence 10-8 Phosphorescence > 10-4 Internal/External Conversion Variable Note: The rate of vibrational relaxation >> rate of fluorescence/phosphorescence. Therefore: fluorescence/phosphorescence always occurs from the lowest vibrational level of an excited state. 2 Energy of fluorescence ≤ Energy of absorption λ of fluorescence/phosphorescence ≥ λ of absorption Two types of fluorescence/phosphorescence spectra can be obtained: Excitation spectrum Emission spectrum How does one decide on the instrumental conditions (i.e. excitation, emission wavelengths) when acquiring a fluorescence/phosphorescence spectrum? 1. Obtain an absorbance spectrum. Why? 2. Fix excitation monochromator to top of most intense absorbance and scan the emission monochromator to get an emission spectrum. Why? 3. Fix emission monochromator at wavelength of maximum emission, scan the excitation monochromator to get an excitation spectrum. Why? 4. Fix excitation monochromator at wavelength of maximum emission for excitation spectrum and obtain emission spectrum. Why? 3 This 4-step process is not foolproof, but reasonable. Alternatively obtain emission spectra at all excitation wavelengths defined by absorbance spectrum. For most molecules excitation (absorption) does not result in emission (luminescence). Most molecules have a low quantum yield. Structural features which enhance the probability of fluorescence, or increase the quantum yield, include aromaticity and structural rigidity. These factors decrease the rate of radiationless deactivation processes such as internal and external conversion, allowing luminescence to occur. Quantum Yield = Φ = Since Φ = 0 for most molecules, luminescence spectrometry is highly selective (derivitization), but not generally applicable like absorption spectrometry. Molecular luminescence spectrometry is similar to atomic emission in 2 ways: 1) Very low detection limits due to the way the signal is generated 2) The signal is proportional to the number of excited state molecules. How does analyte concentration effect fluorescence intensity? The luminescence intensity is proportional to the incident source power to get the most excitation. Equivalent to temperature in atomic emission. 4 A high intensity arc source is used in luminescence spectrometry, but a lower intensity W lamp is used in absorption spectrometry since the signal is not proportional to source output in absorbance spectrometry. According to the above equation the signal intensity is logarithmically proportional to concentration. But if the absorbance (ЄbC) < 0.05, then 1 – 10-ЄbC ~ ЄbC So in dilute solution, the luminescence signal intensity = PoЄbCΦ = kC For example, if Є = 104 (high but not uncommon), then what is the maximum possible linear concentration, assuming a 1 cm pathlength cell? It is strange but at higher concentrations the fluorescence intensity can decrease. Common causes for this include selfquenching and self-absorption. 5 To compare molecular UV-Vis absorption to molecular luminescence spectrometry UV-Vis Fluorescence Detection Limits (M) 10-7 – 10-8 10-11 – 10-12 Selectivity Low High The low selectivity for UV-Vis means it is more generally applicable, but interferences are a larger problem. Chapter 15 Questions/Problems 1-3, 5, 9, 13 6