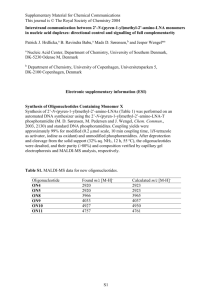
Immunolabling 2
advertisement

Announcements, Week 6 • • • • Happy Darwin Day! (b. Feb. 12, 1809) BPAE cell reports due today. Paper discussion (Becky): Garcia-Pichel et al. 2001 TBA this week: Drosophila embryos, mouse intestine cryostat sections – Reports due Feb. 19. – TBA for Group 4 following lecture. • Week 7, Feb. 19: Fluorescent probes, live cell labeling – TBA: onion epithelium, report due Feb. 26 – Paper discussion next week (Rachel): Tan et al. 2005. • Week 8, Feb. 26: Midterm exam on lecture, lab material; microscope checkout (change from end of semester) during TBA time – allow 30 minutes each – Project descriptions also due. Lecture Outline A. Immunolabeling (cont’d) 1. General considerations 2. Autofluorescence and background 3. Controls B. Fluorescence 1. 2. 3. 4. Defined Absorption and emission spectra Filters Cross-over (bleed-through) compensation C. TBA: Drosophila and mouse intestine samples Detection Methods Method Advantages Disadvantages Recommended for Fluorescence High resolution Doubling labeling possible Staining live cells possible Requires special microscope High resolution studies Double labeling Enzyme High sensitivity Only need bright field light microscopy Permanent Low resolution Endogenous enzyme activities Double staining difficult Substrate toxicity Low resolution studies Rapid antigen screens From: Harlow and Lane, 1999 Antibody Choice Polyclonal antibodies Monoclonal antibodies Pooled monoclonal antibodies Signal strength Excellent Fair Excellent Specificity Good, but some background Excellent, but some crossreactions Excellent, by avoiding any antibodies with cross reactions Good features Signal strength Specificity Signal strength and specificity Bad features Background Often need to titre Lower signal strength Availability From: Harlow and Lane, 1999 Immunolabeling of live cells • Commonly used for surface antigens. • Cellular endocytosis can be used to take up antibody. • Streptolysin-O (SLO) can be used to permeabilize cells and retain viability. Troubleshooting • Problem 1: No specific staining with single labeling • Possible causes: – Antigen not cross reactive, destroyed or inaccessible • Perform positive control, obtain fresh material, permeabilize – Primary antibody dead or not concentrated enough • Obtain fresh or increase concentration – Secondary antibody dead or not concentrated enough • Obtain fresh or increase concentration – Protocol errors • Review protocol, modify • Problem 2: Artifactual staining: do neg. controls. Autofluorescence • Autofluorescence is caused by the intrinsic properties of some structures, independent of antibody labeling. – Aromatic amino acids and other molecules containing ring structures – Chitin, chlorophyll, collagen, elastin – Often worse with shorter wavelength excitation that with longer. – Aldehyde cross-linking (especially gluteraldehyde), methanol fixation • Low level of autofluorescence may be helpful to see limit of cells or tissues. • Negative control with no fluorescent probe determines location and limits of autofluorescence. Background staining • Caused by binding of antibody. • Nonspecific background: Binding of antibodies by parts other than antigen-binding site – Spin down prior to use to remove large particles – Titrate concentrations to minimum – Use blocking reagents, e.g. BSA, nonfat dry milk, normal serum from same species as labeled antibody – Use detergent in all solutions – Reduce incubation times, increase wash times and numbers • Specific background: Caused by antigen binding in side reactions – Dilute primary antibody in 1% normal serum from same species as labeled antibody Controls for immunofluorescence labeling • Spectral properties of the available dyes limit the experimental freedom. • Often it is even difficult to clearly separate two fluorescence markers. • With more markers, the problem grows increasingly complex. Crossover Controls for Double Labeling (Indirect Immunofluorescence) • Experimental: – Mouse Primary 1 + anti-mouse IgG secondary-FL 1, e.g. mouse anti-tubulin + goat anti-mouse-rhodamine. – Rabbit Primary 2 + anti-rabbit IgG secondary-FL 2, e.g. rabbit anti-actin + goat anti-rabbit-fluorescein. • Negative Controls for Primary Specificity: – Pre-Immune Serum or non-immune serum from mouse + Anti-mouse IgG secondary antibodyrhodamine. – Pre-immune Serum or non-immune serum from rabbit + Anti-rabbit IgG secondary antibody-fluorescein. Controls for Double Labeling, Indirect Immunofluorescence (cont’d) • Negative Controls for Secondary Specificity: – Primary 1 + Secondary 2, Primary 2 + Secondary 1 – Look for secondaries that say “no cross-reactivity” • Staining with one antibody and another non-antibody probe (e.g. rhodamine-phalloidin or Sytox Green) is a simpler matter. • If you want to use two monoclonal antibodies, one solution is to conjugate fluorochromes to them directly, e.g. – mouse anti-tubulin-rhodamine – mouse anti-actin-fluorescein • Sequential staining is also possible, fixing between steps. • Or another way is: Fab Fragments for Blocking and Double Labeling of Primary Antibodies from the Same Host Species (Jackson ImmunoResearch Laboratories, Inc.) Key: Rabbit anti-Antigen X Rabbit anti-Antigen Y Fluorescein (FITC) Fab fragment Goat anti-Rabbit IgG (H+L) Rhodamine Red-X (RRX) Goat anti-Rabbit IgG (H+L) See alternative methods at www.jacksonimmuno.com Imaging Double-Labeled Samples • Select dyes that that are well-separated in their absorption and emission spectra. – E.g. use Texas Red (596-620) instead of rhodamine (550-580) with fluorescein (490520). • Be careful about turning up the gain: you can make almost anything “fluorescent.” • Compensate for fluorescence cross-over (discussed below). What is fluorescence? Single-photon excitation • Where a molecule emits light at a specific wavelength when irradiated by light of a shorter wavelength. • Jablonski diagram depicts molecular events of singlephoton fluorescence: 2. excited lifetime 3. emission 1. absorption Fluorescence: Multi-photon excitation • 2 longer (infrared) wavelength photons absorbed simultaneously, emitting a shorter wavelength photon. • Advantage of multiphoton confocal microscope is that thicker samples can be penetrated, compared to UV or visible light. Absorption and Emission Spectra: Spectral overlap and Stokes shift • Spectral overlap must be eliminated by filters, otherwise brighter excitation light will overwelm dimmer emission light. • The bigger the Stokes shift, the easier it is to separate excitation from emission. Reduced quantum yield using suboptimal excitation wavelength • • • Quantum yield is a measure of the efficiency of conversion of absorbed light into emitted fluorescence. Same green light is emitted from purple versus blue excitation, just dimmer with purple excitation. Local environment, e.g. protein conjugation, pH, can also affect absorption spectrum and therefore quantum yield. Filters and Dichroic Mirrors • Dichroic mirrors – Reflect some wavelengths – Transmit other wavelengths • Excitation and Emission (Barrier) filters – Absorbs some wavelengths, transmits others • short pass allows transmission below cutoff • long pass allows transmission above cutoff • narrow band pass allows a range to be transmitted Excitation filters, dichroic mirrors and emission filters Absorption and emission spectra, FITC and rhodamine, with long pass 510, 565 filters 490 520 550 580 BA510IF, BA530RIF – CH 1 FITC only Rhodamine only or CH 2 2-channel imaging, using long pass 510 + short pass 530 (= narrow band pass), long pass 565 filters Typical Crossover Problem: FITC and rhodamine (TRITC) emission spectra 570 cutoff: Ch. 1 Ch. 2 Solution 1: Cut off FITC emission tail using OFFSET in Acquire panel or attenuate laser power Solution 2: Collect 2 channels sequentially “Bleed-through” of FITC into Ch. 2 “Bleed-through” of TRITC into Ch. 1 400 500 600 700 Fluorescence Tutorials from Invitrogen/Molecular Probes • Basic Fluorescence: http://probes.invitrogen.com/resources/educatio n/tutorials/1Introduction/player.html • Spectra: http://probes.invitrogen.com/resources/educatio n/tutorials/2Spectra/player.html • Filters: http://probes.invitrogen.com/resources/educatio n/tutorials/3Light_Sources_Filters/player.html (a-c) AlexaFluor 488 and Cy3 simultaneous scanning: live samples require (d-f) AlexaFluor 488 and Cy3 sequential scanning: possible w/ fixed samples Sequential Scanning Java Tutorial: Crossover compensation • http://www.olympusconfocal.com/theory/bleedthrough.html • Java tutorial: http://www.olympusconfocal.com/java/crossoversimulator/index.html Minimizing crossover: specimen labeling precautions (Molecular Expressions) • Choose fluorochromes with as widely separated spectra as possible. • Adjust concentrations of fluorescent stains so that intensities are close to equal • When selecting fluorescent probes for multiplylabeled specimens, the brightest and most photostable fluorophores should be reserved for the least abundant cellular targets. Minimizing crossover: instrumental approaches (Molecular Expressions) • Absorption spectra are generally skewed towards shorter wavelengths whereas emission spectra are skewed towards longer wavelengths. • For this reason, multicolor fluorescence imaging should be conducted with the reddest (longest wavelength peak emission) dye imaged first, using excitation wavelengths that are only minimally absorbed by the skewed spectral tails of the bluer dyes. Emission only Balancing emission intensities reduces much crossover Controls for Double Labeling • Background control: specimen without secondary antibody or fluorochrome – Controls for autofluorescence • Bleed-through controls: specimens labeled with each fluorochrome separately. To determine maximum gain before bleed-through: 1. Image green-labeled sample w/488 in Ch. 1, look for cross-over in Ch. 2. 2. Image red-labeled sample w/543 in Ch. 2, look for crossover in Ch. 1. 3. Using these settings, image double-labeled sample (same stain concentrations as above) using sequential scan. Quantum Dots • Quantum dots are semi-conductor nanocrystals coated with inert polymer to which biomolecules can be attached, e.g. antibody. • Advantages: – – – – Less photobleaching High quantum yield Narrow, symmetrical emission spectra means less spectral overlap. Various colors can be excited by same laser line TBA this week 1. Use your stained fly slides to collect Z-series of (a) lower mag overview (lastname 4A), and (b) high mag detail (lastname 4B), (c) negative control (low mag, lastname 4C). • • 2. Use references on reserve in library to identify stages and structures stained. Save images in Week 6 folder and turn in a report next Monday (see new format). Mouse intestine cryostat section (16 um) • • Collect 2-channel image using (a) simultaneous imaging (lastname 5A) and (b) sequential imaging (lastname 5B). Compare dual-labeled samples to controls • Submit two reports, one for each sample.