Electrochemistry-11
advertisement

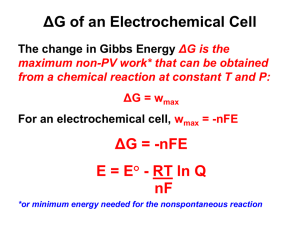
Chemistry Session Electrochemistry - 3 Session Objective • Nernst equation • Equilibrium constant and Nernst equation • Primary cell(Batteries) • Secondary cell(Batteries) • Fuel Cell • Corrosion and its prevention. Nernst Equation For a general reduction reaction, M n ne M(s) The Nernst equation can be written as RT 1 0 E n+ =E - 2 .3 0 3 lo g n+ n+ M /M nF M /M [M ] 0 .0 5 9 1 o E n+ = E lo g M /M Mn+ /M n M n+ (At 298K) Where n = Number of electrons involved [Mn+] = molar concentrations at 298K Illustrative Example Calculate the electrode potential at a copper electrode dipped in a 0.1M solution of copper sulphate at 250C . The standard potential of Cu2+/Cu system is 0.34 volt at 298 K. Solution: Cu2+ + 2e- Cu W e k n o w th a t E Cu 2 / Cu P u ttin g th e v a lu e s o f E E re d 0 .3 4 0 .0 5 9 1 2 E 0 re d Cu 2 / Cu 0 .0 5 9 1 n lo g1 0 [C u 0 .3 4 V , n 2 a n d [C u lo g1 0 [0 .1] 0.34 0.02955 ( 1) 0 .3 1 0 4 5 v o lt 0 2 2 ] ] 0 .1 M Equilibrium constant from Nernst equation Consider the following cell reaction in equilibrium then Z n (s) + C u 2+ (aq) Zn 2+ + C u (s) 0 E = 0 = E (cell) 0 E (cell) = 2.303RT 2.303RT 2F 2F log 2+ Therefore, Generally, ] 2+ [Cu ] 0 [Zn E (cell) = ] 2+ [Cu ] ] 2+ [Cu ] = Kc E (cell) = 0 log 2+ [Zn At equilibrium 2+ [Zn 2.303RT 2F 2.303RT 2F logK c logK Electrode potential Then Nernst equation is: Reduction half reaction Equilibrium point Oxidation half reaction Progress of reaction Illustrative Example Calculate the equilibrium constant of the reaction: Cu(s)+2Ag+(aq.)Cu2+(aq.)+Cu(s) E0=0.46 V Solution : 0 E (ce ll) = lo g K c = 0 .0 5 9 2 lo g K c 0 .4 6 × 2 0 .0 5 9 Kc = 4 × 10 15 = 1 5 .6 Electrochemical cell and Gibbs Energy of the reaction Δ r G = -n F E e .g . fo r ce ll re a ctio n Z n (s) + C u 2+ (a q .) Z n 2+ (a q .) + C u (s) Δ r G = -2 F E If th e re a cta n ts a re in sta n d a rd sta te th e n , 0 Δ rG = Δ rG , E = E Δ rG 0 = -n F E 0 = -n F × Δ rG 0 0 RT nF = -R T ln K ln K Illustrative Example Calculate DG° for Zn-Cu cell at standard state conditions [Given Eo 2+/Zn Zn = –0.76 V, E0 2+ Cu /Cu Solution E cell E Cu 2 / Cu E Zn 2 / Zn = + 0.34 V – (–0.76 V) = 1.10 V DG° = –nFE° = 2×96500×1.10 = –212.3 kJ mol–1 = +0.34 V ] Commercial Cells Primary Cell Dry Cell Commercial Cells The oxidation taking place at the negative zinc electrode. Anode: Z n (s) Z n2+ (aq)+ 2 e The reduction takes place at positive electrode Cathode: 2M n O + H O + 2e M n O + 2O H - 2 2 2 - 3 The net cell reaction is Z n(s) 2M n O 2 (s) H 2O Zn 2 M n 2O 3 2O H The emf of the cell is about 1.45 V. Secondary Cells Lead storage battery Net cell reaction is reversible. Hence, it can be recharged. Lead storage battery At anode: 2- Pb s + SO4 a q . PbSO4 s + 2e - At cathode: 2- P b O2 + S O 4 a q . + 4H + a q . + 2e - P b S O 4 s + 2 H2 O Overall reaction: P b s + P b O2 s + 4 H + a q . + 2 S O 24 a q . 2 P b S O 4 s + 2 H 2 O Secondary Cell In the above equation H2SO4 is used up during the discharge.During recharging the reactions are the reverse of those that occurs during discharge. At cathode: P bS O 4 s 2e 2 P b s S O 4 a q. At anode: P b S O 4 s 2H 2 O 2 PbO2 S O 4 a q. 4H a q. 2e Overall reaction: 2P b S O 4 s 2H 2 O P b s P b O 2 s 4H a q. 2S O 24 a q. Fuel Cells Galvanic cells which converts energy of combustion of fuel like hydrogen, methane and methanol etc. directly into electrical energy are called fuel cells. Example One of the most successful fuel cell uses hydrogen and oxygen reaction to form water. At cathode: O2(g)+2H2O(l)+4e- 4OH-(aq.) At anode: 4H2O(l)+4e- 2H2 + 4OH-(aq.) Overall cell reaction is: 2H2(g)+O2(g) 2H2O(l) Efficiency of fuel cell is 70% much more as compared thermal plants(40%). Corrosion Process of slowly eating away of the metal due to attack of atmospheric gases on the surface of the metal. Examples of corrosion • Rusting of iron • Tarnishing of silver • Development of green coating on copper and bronze, etc. Corrosion Methods of preventing corrosion • Barrier protection • Using anti rust solutions • Sacrificial protection For example iron surface is covered with a metal which has higher tendency to get oxidized (larger negative value of standard reduction potential) than iron. Zinc is used for covering iron and the process is called galvanization. Illustrative Example The standard reduction potentials of Sn+2/Sn and Zn+2/Zn are respectively –0.14V, -0.76V. Predict whether the corrosion of tin can be prevented by coating with zinc or not. Solution : Zinc lies above tin in the electrochemical series, therefore it has a lower reduction potential than tin.This property is employed to prevent corrosion of tin by coating it with zinc as zinc acts as a sacrificial electrode. Thank you