Gibbs Energy & Nernst Equation: Electrochemical Cell EMF
advertisement

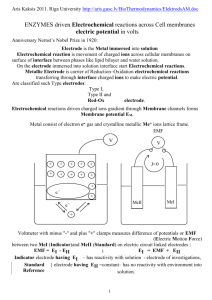
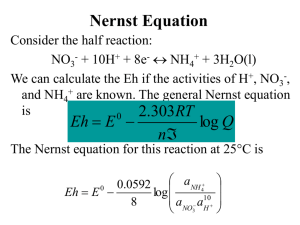
ΔG of an Electrochemical Cell The change in Gibbs Energy ΔG is the maximum non-PV work* that can be obtained from a chemical reaction at constant T and P: ΔG = wmax For an electrochemical cell, wmax = -nFE ΔG = -nFE E = E° - RT ln Q nF *or minimum energy needed for the nonspontaneous reaction The Nernst Equation – Example 1 E = E° - 0.0592 V log Q n What is the emf of the following cell at 298 K? This is called a concentration cell. Zn2+(aq, 0.500 M) Zn2+(aq, 0.150 M) Q = 0.150 0.500 = 0.300 M E = 0 - 0.0592 log .300 = 0.015 V 2 The Nernst Equation – Example 2 Find the solubility product for silver thiocyanate at 25°C, given AgSCN(s) + e- Ag(s) + SCN-(aq) E°(25°C) = 0.0895 V and using the standard reduction potential for Ag+ found in Appendix E. Answer: KSP = 1.0 x 10-12 The Nernst Equation – Example 3 The concentration of potassium ions in the intracellular fluid (ICF) is 135 mM. In the extracellular fluid (ECF), it is 4 mM. What potential must be applied to the cell to keep the K+ from diffusing out of the cell? Answer: The ECF must have a potential of 94 mV relative to the ICF in order to keep the K+ from diffusing out of the cell. The Nernst Equation – Example 4 In a galvanic cell with two hydrogen electrodes, both at 298 K, the first electrode has PH2 = 1.00 bar and an unknown concentration of H+. The second hydrogen electrode is the SHE. Ecell is found to be 0.211 V and the electron flow is from the first electrode to the second. What is the pH of the solution in the first hydrogen electrode? Answer: pH = 3.56 The Nernst Equation – Example 5 In a galvanic cell at 25°C, the cathode halfreaction is Ag+(aq, 1M) + e- Ag(s). The anode is a hydrogen electrode with PH2 = 1.00 bar. It is in a buffer containing 0.10 M benzoic acid and 0.050 M sodium benzoate. Ecell(25°C) = 1.030 V. What is the pKa of the benzoic acid? Answer: pKa = 4.20 The Nernst Equation – Example 6 4Fe2+(aq) + O2(g)+ 4H+(aq) 4Fe3+(aq) +2H2O(l) What is the cell emf at 25°C when [Fe2+]=1.3M, [Fe3+]=0.010M, PO2 = 0.5 bar, and pH=3.50? E = Eº - .0592 log Q …need n, Q, and Eº n Answer: E = 0.37V The Nernst Equation – Example 7 2Fe3+(aq) + H2(g) 2Fe2+(aq) + 2H+(aq) What is the cell emf at 25°C when [Fe2+]=0.0010M, [Fe3+]=2.50M, PH2 = 0.85 bar, and pH=5.00? E = Eº - .0592 log Q …need n, Q, and Eº n Answer: E = 1.27V