Lab report for expt. 21H
advertisement

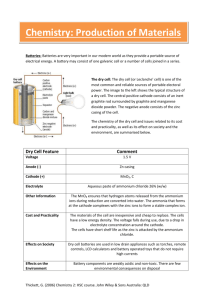
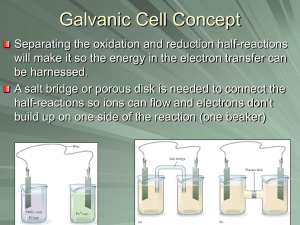
Name: _________________________________ Partner: _______________________________ Experiment 21H Electrochemistry: Corrosion DATA ATMOSPHERIC CORROSION Step 1: In a single 400 mL beaker, set up a cell as described in the lab (zinc and copper electrodes in pure water). Measured potential _________________ V Measured current ________________ mA Which metal is acting as the cathode (red wire)? ________________ Which metal is acting as the anode? ________________ Step 2 (Observations after adding phenolphthalein.): (Note: When the multimeter reads voltage, no current flows so no reaction occurs. When the multimeter reads current, the reaction takes place.) After a minute or two (when reading current), you may see a color change somewhere in the cell. If you see a color change, describe what you see and where you see it. If you don’t see a color change, simply say so. Step 3: Repeat the last experiment using 0.50 M NaCl instead of water. Measured potential _________________ V Measured current _________________ mA Observations after adding phenolphthalein (on current setting): Describe the color change and where you see the color forming inside the cell. 1 Step 4: Set up the cell as described in the lab (Two zinc electrodes in salt water.) Initial potential ________________ V Initial current _________________ mA (Both should be about zero.) What happens to the potential and current while squeezing bubbles near one electrode? What happens to the potential and current while squeezing bubbles near the other electrode? (notice signs!) (Note: The voltage and current changes will be quite small but noticeable) Step 5 (Observation after adding phenolphthalein): Continue to blow bubbles near each electrode and look for a color change. Describe where you see the color relative to where you are blowing the bubbles. 2 CHEMICAL CORROSION AND “GALVANIC PROTECTION” (aka “CATHODIC PROTECTION”) Steps 6-11 (iron nail and zinc strip immersed in HCl): Mass of iron nail before reaction (g) Mass of iron nail after reaction (g) Unconnected (steps 6-8) Do you see bubbles forming near the nail? Reaction that explains bubbles: Change in mass (g) Mass of zinc strip before reaction (g) Mass of zinc strip after reaction (g) Do you see bubbles forming near the zinc? Reaction that explains bubbles: Change in mass (g) Mass of iron nail before reaction (g) Effect of wire on amount of bubbling near the iron nail: Mass of iron nail after reaction (g) Connected (steps 9-11) Change in mass (g) Mass of zinc strip before reaction (g) Effect of wire on amount of bubbling near the zinc: Mass of zinc strip after reaction (g) Change in mass (g) 3 IMPRESSED VOLTAGE FOR CORROSION PROTECTION Steps 12-15 (2 nails submerged in NaCl and connected to power supply): Mass before reaction (g) Nail 1 (connected to + terminal of power supply) Bubbling?: Mass after reaction (g) Change in mass (g) Mass before reaction (g) Nail 2 (connected to – terminal of power supply) Bubbling?: Mass after reaction (g) Change in mass (g) 4 DATA ANALYSIS ATMOSPHERIC CORROSION Step 1: Zinc and copper electrodes in pure water Write the equation and the standard reduction potential for the for the half-reaction the occurred at the anode. Anode (oxidation) half-reaction: Eoanode = Initially, there are no metal ions dissolved in the water so what is being reduced at the cathode? There are really only two possibilities. Find the two possible reduction half-reactions in appendix E (attached) and write them below along with their standard reductions potentials (Eo). Be careful, there are two reactions that may look correct to you but are impossible because one of the reactants is missing! (Let me check your answers!) Reduction 1: Eocathode = Reduction 2: Eocathode = Which of the two substances is reduced more easily? (This is the reduction with the most positive Eo.) Which substance is the stronger oxidizing agent? (Same as the previous answer!) Show that the reaction of zinc with the stronger oxidizing agent is spontaneous (under standard conditions) by calculating the standard cell potential. (Refer to the sign of Eocell.) Reduction: Eocathode = Oxidation: Eoanode = Overall: Eocell = Show that the weaker oxidizing agent can NOT oxidize zinc (under standard conditions) by calculating the standard cell potential. Reduction: Eocathode = Oxidation: Eoanode = Overall: Eocell = 5 Compare the calculated standard cell potential for the spontaneous reaction to your measured cell potential. Should they be the same? Why or why not? Step 2 (Observations after adding phenolphthalein). Based on your proposed reductions (above), why should the solution eventually turn pink? Step 3. Zinc and copper electrodes in 0.50 M NaCl. You should have seen pink forming near one of the electrodes. Was this the anode or the cathode? Explain why the pink color should have formed at this electrode. The rate of color formation and the measured currents showed that the reaction is much faster in 0.50 M NaCl than it was in pure water. Why is the reaction faster? (Hint: Electrons can’t swim!) Draw and label this cell (include NaCl). Label the anode and cathode. Show the movement of electrons and ions. Show each half reaction as it occurs at each electrode. 6 Step 4. Two zinc electrodes in salt water Draw and label this cell. Show oxygen bubbles being blown near one of the electrodes. (Is this the anode or the cathode?) Label the anode and cathode. Show the movement of electrons and ions. Show each half reaction as it occurs at each electrode. (The half reactions are identical to those occurring in the previous cell.) Step 5 (Observation after adding phenolphthalein). You should have seen the pink color form at the electrode at which you blew bubbles? Why? 7 CHEMICAL CORROSION AND “GALVANIC PROTECTION” (aka “CATHODIC PROTECTION”) The term “chemical corrosion” is used when H+ (from an acid) is the oxidizing agent. (“Atmospheric corrosion” is used when O2 is the oxidizing agent.) Use the half-reaction method to write the balanced net-ionic equation for the reaction between iron and HCl (H+). Calculate the standard cell potential. Do the same for the reaction between Zn and HCl. (Zinc and iron both lose 2 electrons.) Reaction between iron and HCl: Reduction: Eocathode = Oxidation: Eoanode = Overall: Eocell = Reaction between zinc and HCl: Reduction: Eocathode = Oxidation: Eoanode = Overall: Eocell = Should these reactions be spontaneous (under standard conditions)? Find a metal that can NOT be corroded by H+ (under standard conditions) by showing that the reaction would have a negative standard reduction potential. Reduction: Eocathode = Oxidation: Eoanode = Overall: Eocell = 8 Steps 6-11 (iron nail and zinc strip immersed in HCl) The results demonstrate the phenomenon of “galvanic protection” (also called “cathodic protection”). When two metals are electrically connected and placed in contact with an oxidizing agent, the more active metal becomes the anode of an electrochemical cell and the less active metal becomes the cathode. a. Why is the more active metal called a “sacrificial anode”? b. Draw and label a diagram of the unconnected zinc strip and nail immersed in HCl. Show bubbles forming. Show the half-reactions and overall reactions occurring at each metal. (Oxidation and reduction occurs at both metals.) c. Draw and label a diagram of the cell that resulted from connecting the nail to zinc and immersing in HCl. Label the cathode and anode. Show the movement of electrons and ions. Show the production of gas (only at the cathode). Show the half-reactions occurring at each electrode. 9 Steps 12-15: Impressed Voltage for Corrosion Protection (2 nails submerged in NaCl and connected to power supply) In the “impressed voltage” experiment, there are two reduction half-reactions occurring at the cathode: The reduction of oxygen as before and the reduction of water which explains the formation of bubbles! (The reduction of water did not occur in any of the previous cells.) Remember there is no H+ in the cell! Write the equations for the half-reactions and calculate the standard cell potential corresponding to each of the two overall cell reactions. Both of the oxidation half-reactions are the same (oxidation of iron). Reduction: Eocathode = Oxidation: Eoanode = Overall: Eocell = Reduction: Eocathode = Oxidation: Eoanode = Overall: Eocell = One of the standard cell potentials is negative. Why does this reaction occur in this experiment? The power supply provides a source of electrons. Electrons emerge from the (-) terminal of the power supply and enter at the (+) terminal. Draw and label a diagram of this experiment. Include the power supply and indicate the signs of the terminals to which the wires were connected. Show the movement of electrons and ions (Na+ and Cl-). Write the half-reaction below each electrode. Show both reduction reactions occurring at the cathode. Show bubbles forming at the cathode. Briefly explain why only one of the two nails was corroded. 10 11