Acid-base titrations
advertisement
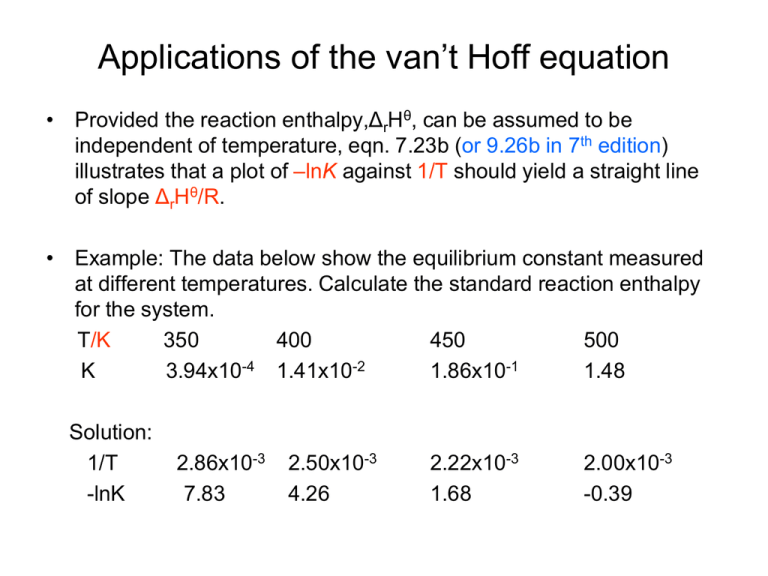
Applications of the van’t Hoff equation • Provided the reaction enthalpy,ΔrHθ, can be assumed to be independent of temperature, eqn. 7.23b (or 9.26b in 7th edition) illustrates that a plot of –lnK against 1/T should yield a straight line of slope ΔrHθ/R. • Example: The data below show the equilibrium constant measured at different temperatures. Calculate the standard reaction enthalpy for the system. T/K 350 400 450 500 K 3.94x10-4 1.41x10-2 1.86x10-1 1.48 Solution: 1/T -lnK 2.86x10-3 7.83 2.50x10-3 4.26 2.22x10-3 1.68 2.00x10-3 -0.39 Continued • Self-test 7.5: The equilibrium constant of the reaction 2SO2(g) + O2(g) ↔ 2SO3(g) is 4.0x1024 at 300K, 2.5x1010 at 500K, and 2.0x104 at 700K. Estimate the reaction enthalpy at 500K. Solution: discussion: 1. Do we need a balanced reaction equation here? 2. What can be learned about the reaction based on the information provided? 3. Will the enthalpy become different at 300K or 700K? Calculate the value of K at different temperatures • The equilibrium constant at temperature T2 can be obtained in terms of the known equilibrium constant K1 at T1. • Since the standard reaction enthalpy is also a function of temperature, when integrating the equation 9.26b from T1 to T2, we need to assume that ΔrHө is constant within that interval. 1 K2 K T2 d ln( K ) 1 rH d( R 1 1 ) T T1 • so ln(K2) – ln(K1) = rH R ( 1 T2 1 ) (7.24) T1 • Equation 7.24 provides a non-calorimetric method of determining standard reaction enthalpy. (Must keep in mind that the reaction enthalpy is actually temperature-dependent!) Example, The Haber reaction N2(g) + 3H2(g) ↔ 2NH3(g) At 298 K, the equilibrium constant K = 6.1x105. The standard enthalpy of formation for NH3 equals -46.1 kJ mol-1. What is the equilibrium constant at 500K? Answer: First, calculate the standard reaction enthalpy, ΔrHө, ΔrHө = 2*ΔfHө(NH3) - 3* ΔfHө(H2) - ΔfHө(N2) = 2*(-46.1) – 3*0 - 1*0 = - 92.2 kJ mol-1 then 1 ln(K2) – ln(6.1*105) = 8 .3145 *(-92.2*1000 J mol-1) (1/500 – 1/298) ln(K2) = -1.71 K2 = 0.18 • Despite the decrease in equilibrium constant as a result of temperature increase, yet in industrial production it is still operated at an elevated temperature (kinetics vs thermodynamics) Practical Applications of the Knowledge of the temperature dependence of the equilibrium constant (i) M(s) + 1/2O2(g) → MO(s) (ii) 1/2C(s) + 1/2O2(g) → 1/2CO2(g) (iii) C(s) + 1/2O2(g) → CO(g) (iv) CO(g) + 1/2O2(g) → CO2(g) • This is carried out based on two criteria (1) Gibbs energy is a state quantity and thus can be added or subtracted directly. (2) When the standard reaction Gibbs energy is negative, the forward reaction is favored (i.e. K > 1). Standard reaction Gibbs energy is sometimes referred as Free Gibbs energy. Equilibrium Electrochemistry Thermodynamic functions of ions in solution (10.1 & 2 of 7th edition or 5.9 in 8th edition) • The standard enthalpy and Gibbs energy of ions are used in the same way as those for neutral compounds. • Cations cannot be prepared without their accompanying anions. Thus the individual formation reactions are not measurable. • Defining that hydrogen ion has zero standard enthalpy and Gibbs energy of formation at ALL temperature. ΔfHθ(H+, aq) = 0; ΔfGθ(H+, aq) = 0 • The standard Gibbs energy and enthalpy of formation for other ions can be calculated in relative to the value of hydrogen ion. • Consider: ½ H2(g) + ½ Cl2(g) H+(aq) + Cl-(aq) ΔrGθ = -131.23kJ mol-1 (this can be obtained from K) ΔrGθ = ΔfGθ(H+, aq) + ΔfGθ(Cl-, aq) – ½ ΔfGθ(H2, g) + ½ ΔfGθ(Cl2, g) = 0 + ΔfGθ(Cl-, aq) – 0 – 0 = ΔfGθ(Cl-, aq) therefore the standard Gibbs energy of formation for Cl- ion can be obtained from the standard Gibbs energy of reaction. Standard Gibbs energy and enthalpy of formation of other ions can be achieved through the same approach. • Now, consider: Ag(s) + ½ Cl2(g) Ag+(aq) + Cl-(aq) ΔrHθ = ΔfHθ(Ag+, aq) + ΔfHθ(Cl-, aq) - 0 - ½ *0 ΔrGθ = ΔfGθ(Ag+, aq) + ΔfGθ(Cl-, aq) - 0 - ½ *0 (Once the standard reaction Gibbs energy is calculated, the calculation of the equilibrium constant will be the same as discussed for neutral solutions) Thermodynamic cycles (chapter 3.6, 8th edition) • The sum of the Gibbs energy for all steps around a circle is ZERO! • The Gibbs energy of formation of an ion includes contributions from the dissociation, ionization, and hydration. • Gibbs energies of solvation can be estimated from Max Born equation. 2 2 solv G zi e N A 1 1 8 0 ri r where zi is the charge number, e is the elementary charge, NA is the Avogadro’s constant, ε0 is the vacuum permittivity, εr is the relative permittivity, ri is ion’s radius. • ΔsolvGθ is strongly negative for small, highly charged ions in media of high relative permittivity. • For water at 25oC: 2 solv G zi ( ri / pm ) 6 .86 10 4 kJmol 1 • Example (Self-test 10.2 7th edition): Estimate the value of ΔsolvGө(Br-, aq) - ΔsolvGө (Cl-, aq) from the experimental data and from the Max Born equation. Solution: To calculate the difference of their experimental measurement, use the data provided in Table 2.6: ΔsolvGө(Br-, aq) = -103.96 kJ mol-1; ΔsolvGө(Cl-, aq) = -131.23 kJ mol-1; So ΔsolvGө(Br-, aq) - ΔsolvGө (Cl-, aq) = -103.96 – ( 131.23) = 27.27 kJ mol-1; In order to apply the Born equation, we need to know the radius of the corresponding ions. These numbers can be obtained from Table 23.3 r(Br-) = 196 pm; r(Cl-) = 181 pm; thus ΔsolvGө(Br-, aq) - ΔsolvGө (Cl-, aq) = - (1/196 – 1/181)*6.86*104 kJ mol-1 = 29.00 kJ mol-1 (The calculated result is slightly larger than the experimental value). Ion activity and mean activity coefficient • The activity relates to the molality b via α = γ * b/bө where γ is called the activity coefficient and bө equal 1mol kg-1. • Now the chemical potential will be expressed by the following equation: μ =μө + RT ln (b/bө) + RTln (γ) = μideal + RTln (γ) • Consider an electrically neutral solution of M+ X-, G = μ+ + μ- = μ+ideal + μ-ideal + RTln(γ+) + RTln (γ-) =Gideal + RTln (γ+γ-) • Since there is no experimental way to separate the product (γ+γ-) into contributions from the cations and anions, mean activity coefficient γ is introduced here to assign equal responsibility for nonideality to both ions. • The mean activity coefficient γ is calculated as (γ+γ-)1/2 • The chemical potential for individual ions M+ and X- then becomes: μ+ = μideal + RTln (γ) μ- = μideal + RTln (γ) For a general compound of the form, MpXq, the mean activity coefficient is expressed as: γ = [(γ+)p(γ-)q]1/s with s = p+q • • Debye-Hückel limiting law is employed to calculate the mean activity coefficient: log(γ) = -|z+z-| A I1/2 (5.69 in 8th edition) A = 0.509 for aqueous solution at 25oC. I is the ionic strength, which is calculated as the following: I = ½ zi2(bi/bө) (5.70 in 8th edition) where zi is the charge number and bi is the molality of the ion. Example: Relate the ionic strength of (a) MgCl2, (b) Fe2(SO4)3 solutions to their molality, b. Solution: To use the equation 5.70, we need to know the charge number and the molality of each ion: MgCl2: From molecular formula, we can get b(Mg2+) = b(MgCl2); Z(Mg2+) = +2; b(Cl-) = 2*b(MgCl2); Z(Cl-) = -1; So I = ½((2)2*b +(-1)2*(2b)) = ½(4b + 2b) = 3b; For Fe2(SO4)3: From the molecular formula, we get b(Fe3+) = 2*b(Fe2(SO4)3); with Z(Fe3+) = +3; b(SO42-) = 3*b(Fe2(SO4)3); with Z(SO42-) = -2; So I = ½((3)2*(2b) +(-2)2*(3b)) = ½(18b + 12b) = 15b * What is the unit of I ? Solutions contain more than one types of electrolytes • The ionic strength of the solution equals the sum of the ionic strength of each individual compound. Example: Calculate the ionic strength of a solution that contains 0.050 mol kg-1 K3[Fe(CN)6](aq), 0.040 mol kg-1 NaCl(aq), and 0.03 mol kg-1 Ce(SO4)2 (aq). Solution: I (K3[Fe(CN)6]) = ½( 12*(0.05*3) + (-3)2*0.05) = ½ (0.15 + 0.45) = 0.3; I (NaCl) = ½(12*0.04 + (-1)2*0.04) = 0.04; I (Ce(SO4)2) = ½(42*0.03 + (-2)2*(2*0.03)) =0.36; So, I = I(K3(Fe(CN)6]) + I(NaCl) + I(Ce(SO4)2) = 0.3 + 0.04 + 0.36 = 0.7 Calculating the mean activity coefficient Example: Calculate the ionic strength and the mean activity coefficient of 2.0m mol kg-1 Ca(NO3)2 at 25 oC. Solution: In order to calculate the mean activity coefficient with the eq. 5.70, one needs to know the ionic strength of the solution. Thus, the right approach is first to get I and then plug I into the equation (5.70). I = ½(22*0.002 + (-1)2*(2*0.002)) = 3*0.002 = 0.006; From equation 5.70, log(γ±) = - |2*1|*A*(0.006)1/2; = - 2*0.509*0.0775; = -0.0789; γ± = 0.834; Experimental test of the Debye-Hückel limiting law Accuracy of the Debye-Hückel limiting law Example: The mean activity coefficient in a 0.100 mol kg-1 MnCl2(aq) solution is 0.47 at 25oC. What is the percentage error in the value predicted by the Debye-Huckel limiting law? Solution: First, calculate the ionic strength I = ½(22*0.1 + 12*(2*0.1)) = 0.3 to calculate the mean activity coefficient. log(γ) = -|2*1|A*(0.3)1/2; = - 2*0.509*0.5477 = - 0.5576 so γ = 0.277 Error = (0.47-0.277)/0.47 * 100% = 41% Extended Debye-Hückel law • log( ) A | z z | I 1 BI 1/2 1/2 B is an adjustable empirical parameter. Calculating parameter B Example : The mean activity coefficient of NaCl in a diluted aqueous solution at 25oC is 0.907 (at 10.0mmol kg-1). Estimate the value of B in the extended Debye-Huckel law. Solution: First calculate the ionic strength I = ½(12*0.01 + 12*0.01) = 0.01 From equation log( ) A | z z | I 1 BI 1/2 1/2 log(0.907) = - (0.509|1*1|*0.011/2)/(1+ B*0.011/2) B = - 1.67