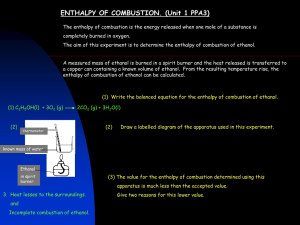
E = c m DT - Our Ladys High School
advertisement
Calculation of Enthalpy Values Using E = c m DT Calculation of DH requires 3 steps 1. Use E = cmDT to calculate the energy change from the experiment 2. A scaling up of this value to obtain the energy change for 1 mole 3. Checking to make sure the sign of the DH is correct Use E = cmDT to calculate the energy change On some occasions this may already be done for you There will be no temperature but there will be a value in kJ The mass of water in kg (litres) Calculate the ofofwater and use for m litreuse weighs 1 kg Do1not thelitres mass chemical thisthis is used in step 2 E = c m DT The change in temperature Specific heat capacity of water be found on the liquid last page of the book It This does will not matter what is, use thedata value for water When 1g of ethanol C2H5OH was burned the heat produced warmed 5litres of water from 20.1 oC to 21.5 oC Calculate the ethalpy of combustion of ethanol E = c m DT 21.5 - 20.1 = 1.4 5 litre = 5kg E = 4.18 x 5 4.18 from databook x 1.4 E = 29.26 kJ Now do step 2 When 1g of ethanol C2H5OH was burned the heat produced warmed 5litres of water from 20.1 oC to 21.5 oC From step 1 E = 29.26 kJ Scale up the value to obtain the energy change for 1 mole 2 x C =Mass 2 x of12ethanol = 24 = 46 g Gram Formula 6 xH = 6 x 1 = 6 1 x O = 1 x 16 = 16 1g 29.26 kJ Gram Formula Mass = 46 g 46g DH 29.26 x 46 1345.96 kJ kJ = 1345.96 kJ Now do step 3 When 1g of ethanol C2H5OH was burned the heat produced warmed 5litres of water from 20.1 oC to 21.5 oC DH From step 2 = 1345.96 kJ Check to make sure the sign of the DH is correct Heat was produced in the reaction making it exothermic DH will have a negative value DH = - 1345.96 kJ mol-1 When 2g of a compound ( formula mass 40) is dissolved in 50 cm3 of water the temperature rises by 10 oC Calculate the enthalpy of solution Step 1 E = c m DT E = 4.18 x 0.05 x 10 E = 2.09 kJ Step 2 2g 40g Step 3 2.09 kJ 41.8 kJ Temperature rise so exothermic DH = - 41.8 kJ mol-1 When asked to calculate the enthalpy of neutralisation some minor changes must be made to the method. a) All liquids, both the acid and alkali volumes, are heated and so are included when calculating the mass of water b) We will not be give a mass of acid We will be given a concentration and a volume we use these to calculate the number of moles of acid used. No. of moles = concentration x volume in litres c) We scale up (or down) the number of moles to 1 When 100cm3 of hydrochloric acid concentration 0.8 mol l-1 is neutralised by 100 cm3 of an alkali, both at 12 oC the temperature of the salt solution rises to 16.6 oC Calculate the enthalpy of neutralisation of hydrochloric acid Step 1 E = c m DT E = 4.18 x 0.2 x 4.6 E = 3.8456 kJ Step 2 No. of moles = conc 0.8 x 0.1 x vol= in0.08 litres 0.08 3.8456 kJ 1 mole Step 3 48.07 kJ Temperature rise so exothermic DH = - 48.07 kJ mol-1