Hess`s Law - Clydebank High School
advertisement
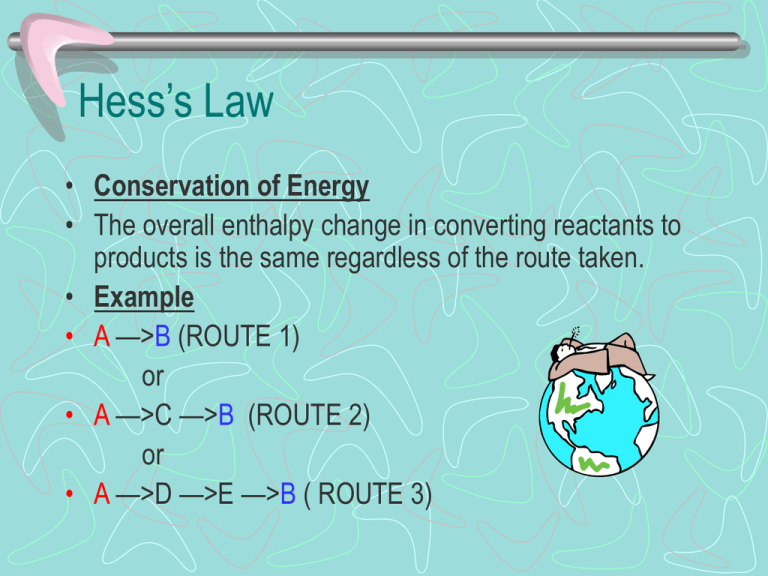
Hess’s Law • Conservation of Energy • The overall enthalpy change in converting reactants to products is the same regardless of the route taken. • Example • A —>B (ROUTE 1) or • A —>C —>B (ROUTE 2) or • A —>D —>E —>B ( ROUTE 3) • • • • ΔH1 = route 1 ΔH2 + ΔH3 = route 2 ΔH4 + ΔH5 = route 3 Hess’s Law states the overall enthalpy change will be the same – therefore: • ΔH1 = ΔH2 + ΔH3 or = ΔH4 + ΔH5 Uses of Hess’s Law • We can use Hess’s Law to calculate enthalpy changes which are very difficult or impossible to do by experiment. • Example • The neutralisation of KOH using HCl. • We can do this by adding KOH(s) directly to HCl(aq) – ΔH1 or Dissolving KOH(s) in water – ΔH2 then Adding KOH(aq) to HCl(aq) – ΔH3 Pathway • • • • • 1. KOH(s) + HCl (aq) —> KCl (aq) + H2O(l) ΔH1 2. KOH(s) + H2O (aq) —> KOH (aq) ΔH2 3. KOH(aq) + HCl(aq) —>KCl (aq) + H2O ΔH3 According to Hess’s Law ΔH1 = ΔH2 + ΔH3 Examples • • • • • • Calculate the energy change for the reaction : RbCl(s) —> Rb+(g) + Cl-(g) Use the following enthalpy changes: RbCl(s) —> Rb+ (aq) + Cl- (aq) + 17 kJ Rb+ (g) —> Rb+ (aq) - 301 kJ Cl- (g) —> Cl- (aq) - 364 kJ The Steps! • 1. Write balanced eq for the reaction you wish to find the enthalpy change. • 2. Write balanced eq for the reactions that have been given to you. • 3. Label each step with an appropriate ΔH. Make sure you have identified more than one route for the reaction to follow. • 4. Write down the enthalpy change for the direct route ( Hess’s Law) • 5. Put in the values for each reaction. 1. RbCl (s) —> Rb+ (g) + Cl- (g) ΔH=? 2. RbCl (s) —> Rb+ (aq) + Cl- (aq) Δ Ha Rb+ (g) —> Rb+(aq) Δ Hb Cl- (g) —> Cl- (aq) ΔHc 3. Route 1 = Δ H ? Route 2 = Δ H a + ( - Δ H b) + (- Δ H c) 4. Hess’s Law Δ H =Δ H a + ( - Δ H b) + (- Δ H c) 5. Δ H = +17 +(+301) + (+364) = + 682 kJ mol/l