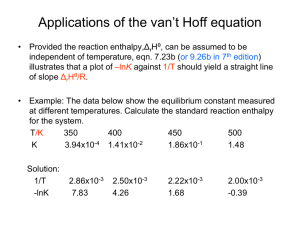
Applications of standard potentials
advertisement
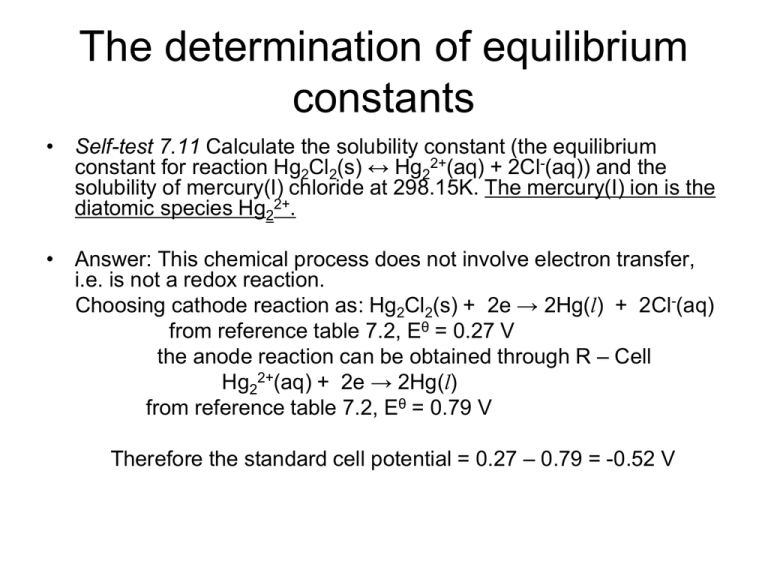
The determination of equilibrium constants • Self-test 7.11 Calculate the solubility constant (the equilibrium constant for reaction Hg2Cl2(s) ↔ Hg22+(aq) + 2Cl-(aq)) and the solubility of mercury(I) chloride at 298.15K. The mercury(I) ion is the diatomic species Hg22+. • Answer: This chemical process does not involve electron transfer, i.e. is not a redox reaction. Choosing cathode reaction as: Hg2Cl2(s) + 2e → 2Hg(l) + 2Cl-(aq) from reference table 7.2, Eθ = 0.27 V the anode reaction can be obtained through R – Cell Hg22+(aq) + 2e → 2Hg(l) from reference table 7.2, Eθ = 0.79 V Therefore the standard cell potential = 0.27 – 0.79 = -0.52 V lnK = Eө*v/25.7mV, here v = 2 lnK = - 40.467 K = 2.62 x 10-18 • K = (aHg(I) * a2cl-)/aHg2cl2 • Using equation = b*(2b)2/1 = 4*b3 = 2.62 x 10-18 therefore b = 8.68 x 10-7 mol/kg Species-selective electrodes: Measuring pH • The half reaction at a hydrogen electrode: H+(aq) + e- → ½ H2(g) 1/ 2 ( f ( H ) / P ) 2 Here v= 1, Q= aH The potential E(H+/H E(H+/H 2) 2) = = Eө - RT ( f ( H 2 ) / P )1 / 2 ln( ) vF aH 1/ 2 ( f ( H ) / P ) RT 2 ln( ) vF aH Assuming that the pressure of H2 gas equals 1 bar E(H+/H 2) = RT 1 - ln( ) vF aH E(H+/H2) = RT ln( a H ) = RT ln(10 ) log a = H F F - RT ln(10) pH F • Two electrodes are required to build up an electrochemical cell. This is why we need a reference electrode when measuring the pH of a solution. • A regular reference electrode is calomel (Hg2Cl2(s)). • The hydrogen electrode is used as the right hand electrode, i.e. cathode. • E(cell) = E(H+/H2) - E(ref.) • E(cell) + E(ref) = - RT ln(10 ) pH F • Why do we need to calibrate the pH electrode before its usage? Thermodynamic function • Nernst equation is a bridge connecting the thermodynamic quantify, Gibbs energy, and the electromotive force. • Consider: Pt(s)|H2(g)|H+(aq)|Ag+(aq)|Ag(s) the ΔfGө(Ag+(aq)). Eө = 0.80V. Calculate Solution: First, write down the two reduction half reactions and then do a simple subtraction (R-L) to get the cell reaction. Cell : R: Ag+(aq) + eL: H+(aq) + eAg+(aq) + 1/2 H2(g) → Ag(s) → 1/2 H2(g) → Ag(s) + H+(aq) Continued ΔrGө = ΔfGө(Ag(s)) + ΔfGө (H+) - ΔfGө (Ag+) - (1/2)ΔfGө(H2(g)) Δ rGө = 0 + 0 - ΔfGө (Ag+) - 0 Since ΔrGө = -νFEө ΔfGө(Ag+) = vFEө = 9.648 x 104 C mol-1 * 0.80V = 7.715 x 104 CV mol-1 = 7.715 x 104 J mol-1 ( 1CV = 1J) Temperature dependence of emf • ΔrGө = -νFEө take the derivate of temperature for both sides d r G vFdE dT dT • dE dT d r G dE dT vF dT is called temperature coefficient of standard cell emf. • Because d r G r S dT S dE one gets r dT vF therefore, one can use electrochemical method to obtain reaction entropy and relate them to entropies of ions in solution. • noncalorimetric method of measuring ΔrHө ΔrHө = ΔrGө + TΔrSө = -vFEө + T(vF dE dT ) = -vF(Eө -T dE dT ) • Example: The standard electromotive force of the cell Pt(s)|H2(g)|H+(aq)||Ag+(aq)|Ag(s) was measured over a broad range of temperatures, and the data were fitted to the following polynomial: Eө/V = 0.07 – 4.11x10-4(T/K – 298) – 3.2x10-6(T/K -298)2 Evaluate the standard reaction Gibbs energy, enthalpy, and entropy at 298K. Solution: The standard reaction Gibbs energy can be calculated once we know the standard emf of the above cell: At 298K, Eө/V = 0.07- 4.11x10-4(298K/K – 298) – 3.2x10-6(298K/K 298)2 Eө/V = 0.07 Eө = 0.07 V • to identify the value of v, we need to write down the cell reaction Ag+ + 1/2H2(g) → H+(aq) + Ag(s) ΔrGө = -vFEө = - (1) x 9.6485 x104 C mol-1 x (0.07V) = - 6.754 x 103 CVmol-1 = - 6.754 x 103 J mol-1 Calculate the temperature coefficient of the cell electromotive force dE dT = – 4.11x10-4 K-1V – 3.2x10-6x2x(T/K -298) K-1V = - 4.11x10-4 K-1V (at 298K) then ΔrSө = vF dE dT = 1 x 9.6485 x 104 C mol-1 x (- 4.11x10-4 K-1V ) = - 39.66 CV K-1 mol-1 = - 39.66 J K-1 mol-1 to calculate the standard reaction enthalpy: ΔrHө = ΔrGө + TΔrSө = -6.754kJ mol-1 + 298K (- 39.66 J K-1 mol-1) = -18.572 kJ mol-1 Example: The standard cell potential of Pt(s)|H2(g)|HCl(aq)| Hg2Cl2(s)|Hg(l)|Ag(s) was found to be +0.269 V at 293 K and + 0.266 V at 303K. Evaluate the standard reaction Gibbs function, enthalpy, and entropy at 298K. Solution: Write the cell reaction Hg2Cl2(s) + H2(g) → 2Hg(l) + 2HCl(aq) So v = 2, To find the ΔrGө at 298 K, one needs to know the standard emf at 298K, which can be obtained by linear interpolation between the two temperatures. Eө = 0.2675 V ΔrGө = -2F Eө = -51.8 kJ mol-1 The standard reaction entropy can then be calculated from dE = (0.266V- 0.269V)/10K = -3.0x10-4 VK-1 dT ΔrSө = 2F dE = - 58 JK-1 mol-1 dT then ΔrHө = ΔrGө + TΔrSө = -69 kJ mol-1 What is the quotient, Q, of the above cell reaction? Evaluate the reaction potential from two others • Example 1. Calculate the standard potential of the Fe3+/Fe from the values for the Fe3+/Fe2+ (+0.77V) and Fe2+/Fe( -0.44V). • Solution: : first write down the half reactions for these three couples: 1) Fe3+ + e- → Fe2+ 2) Fe2+ + 2e- → Fe 3) Fe3+ + 3e- → Fe Reaction 3 is the sum of 1 and 2, yet one cannot use E3 = E1+ E2 ΔrGө(1) = - 1x F x 0.77V ΔrGө(2) = - 2x F x (-0.44)V ΔrGө(3) = ΔrGө(1) + ΔrGө(2) = 0.11F V ΔrGө(3) = - 3*F*E3 = 0.11 F V E3 = -0.033V A good practice of calculating the potential of a redox couple from other redox coupled is going through the reaction Gibbs energy! Example 2: Given that the standard potentials of the Cu2+/Cu and Cu+/Cu couples are +0.340V and + 0.522V, respectively, evaluate Eө(Cu2+, Cu+). Solution: Again, we should go through the standard Gibbs energy to calculate it. First write the half-reactions: (1) Cu2+(aq) + 2e(2) Cu+(aq) + e(3) Cu2+(aq) + e- → → Cu(s) Cu(s) → Cu+ it can be identified easily that reaction (3) equals (1) - (2) thus: ΔrGө(3) = ΔrGө(1) - ΔrGө(2) = (-2*F*0.340V) – (-1*F*0.522V) ΔrGө(3) = -0.158F V -1*F*Eө(Cu2+/Cu+) = -0.158F V Eө(Cu2+/Cu+) = 0.158 V Summary of chapter 7 • • • • • • • • Equilibrium constant, Gibbs energy vs the extent of reaction Reaction Gibbs energy Standard reaction Gibbs energy and thermodynamic equilibrium constant. Le Chaterlier principle & van’t Hoff relationship. Reactions in charged environment: Nerst equation. Cell potential and standard cell potential. Connection between thermal and electrochemical quantities.