幻灯片 1
advertisement

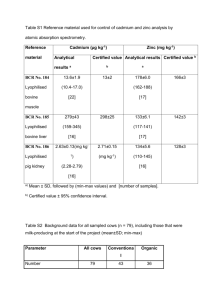
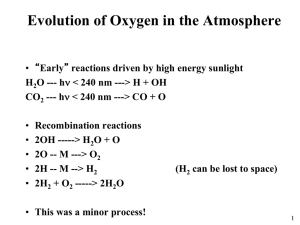
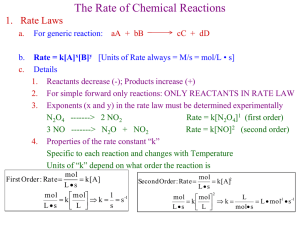
Chapter 7 Electrochemistry §7.5 Theories for strong electrolyte + + + + + + + + + + + + + Self reading: Ira N. Levine, Physical Chemistry, 5th Ed., McGraw-Hill, 2002. pp. 300-304 Section 10.8 the Debye-Hückel theory of electrolyte solutions Empirical formula for electrolyte solution Stationary property Dynamic property ln m m A c A I For ideal solution: For nonideal solution: i i (T ) R T ln m i i i (T ) RT ln m i RT ln i ( G ) T , P RT ln i W r ' ln i Wr ' RT Theoretical evaluation of Wr’ Before 1894: treated solution as ideal gas 1918-1920: Ghosh – crystal structure 1923: Debye-Hückel: ionic atmosphere 1. The Debye-Hückel theory + + + + + + + + + + + + + Ionic atmosphere Radius:1~100 nm Basic assumptions 1) Point charge Peter J. W. Debye 1936 Noble Prize Germany, Netherlands 1884/03/24~ 1966/11/02 Studies on dipole moments and the diffraction of X rays 2) Only coulombic attraction 3) Dielectric constant keep unchanged 4) Boltzmann distribution, Poisson equation Shielded Coulombic potential Z je 4 0 r r Z je 4 0 r r e b / r 1 + 0 r kT 2 b 2 4 N Ie A 0 ln i Debye length ln i RT 1 1 e N A2 02 3 ln i Zi e 2 Wr ' 2 1 Zi I 4 ( 0 r kT ) 2 ln i AZ 2 i I 2 8 0 r kTb Group exercise: Deduce ln A Z Z I Lewis’s empirical equation from ln i AZ 2 i I ln A I for aqueous solution at 298 K lg 0 . 509 Z Z I ln 1 . 172 Z Z I Debye-Hückel limiting law holds quite well for dilute solutions (I< 0.01 m), but must be modified to account for the drastic deviations that occur at high concentrations. lg A ZZ 1 I A ZZ I Valid for c < 0.1 mol·kg-1 lg A ZZ 1 I bI I Valid for c < 1 mol·kg-1 1 I I 2. Debye-Hückel-Onsage theory + + + + + + + + + 1) Relaxation effect 2) Electrophoretic effect In 1927, Lars Onsager pointed out that as the ion moves across the solution, its ionic atmosphere is repeatedly being destroyed and formed again. The time for formation of a new ionic atmosphere (relaxation time) is ca. 10-7 s in an 0.01 mol·kg-1 solution. Under normal conditions, the velocity of an ion is sufficiently slow so that the electrostatic force exerted by the atmosphere on the ion tends to retard its motion and hence to decrease the conductance. 6 41.25( Z Z 2.08 10 Z Z q 3 T ( T ) 2 (1 q ) ) ( ) I Valid for 310-5 ~ 10-3 mol·kg-1 Fuoss: valid for < 0.1 mol·kg-1 Lars Onsager 1968 Noble Prize USA, Norway 1903/11/27~1976/10/05 Studies on the thermodynamics of irreversible processes Falkenhagen: valid for < 5 mol·kg-1, for LiCl: valid until 9 mol·kg-1 I Progress of the theories for electrolyte 1800: Nicholson and Carlisle: electrolysis of water 1805: Grotthuss: orientation of molecules 1857: Clausius: embryo of dissociation 1886: vant’ Hoff: colligative property 1887: Arrhenius: dissociation and ionization 1918: Ghosh: crystalline structure 1923: Debye-Hückel: Debye-Hückel theory 1926: Bjerrum: conjugation theory 1927: Onsager: Debye-Hückel-Onsager theory 1948: Robinson and Stokes: solvation theory Question: Compare the solubility of AgCl(s) in NaCl solution, pure water, and concentrated KNO3 solution using that in pure water as standard. AgCl(s) = Ag+(aq) + Cl¯(aq) Solubility equilibrium and the effect of co-ion. ionic strength on solubility: salt effect