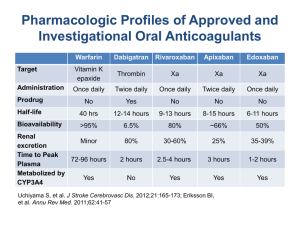
Novel Oral Anticoagulants: Benefits and risks

Novel Oral
Anticoagulants: Benefits and risks
Matthew Moles, MD
December 4, 2012
University of Colorado
1
Objectives
•
Become familiar with the mechanism of action of dabigatran and rivaroxaban
•
Review the data behind the use of
NOACs in Afib and DVT/PE.
•
Evaluate the overall risks across all studies
2
Rivaroxaban
Dabigatran
4
NOACs and Afib
6
7
RE-LY
•
N = 18,113, Follow-up median 2 years, CHADS2 median
2.1, open-label
•
Inclusion: Afib on EKG w/in last 6 months, plus at least one:
CVA, TIA, LVEF < 40%, NYHA class II or great HF symptoms w/in 6 months and age of at least ≥75 or 65-74 plus DM, HTN, or CAD
•
Exclusion: severe heart-valve disorder, stroke w/in 14 days or severe stroke w/in 6 months, increased risk of bleeding,
CrCl < 30, liver dx, prenancy
•
Randomized to 110 or 150 mg of dabigitran BID vs unblinded warfarin (ASA <100 mg or other antiplatelet agents allowed)
•
Primary outcome: stroke or systemic embolization
•
Safety outcome: major hemorrhage (reduction of Hgb by 2 g/dL, 2 units of PRBCs, or symptomatic bleeding in critical area)
8
Event
Stroke/Embolis m
D %/yr
1.11
Stroke
Stroke-
Hemorrhagic
MI
Death from vascular causes
Death any cause
1.01
0.10
0.74
2.28
3.64
W %/yr
1.69
1.57
0.38
0.53
2.69
4.13
D vs W
RR
0.66 (0.53-
0.82)
0.64 (0.51-
0.81)
0.26 (0.14-
0.49)
1.38 (1.00
–
1.91)
0.85 (0.72
–
0.99)
0.88 (0.77
–
1.00)
P value
<0.001
<0.001
<0.001
0.048
0.04
0.051
NNT
172
178
357
476 NNH
243
204
Non-inferiority margin 1.46
9
10
Bleeding Event
Life threatening
Intracranial
GI
NNT/H
285 T
227 T
204 H
11
12
ROCKET-AF
•
N = 14,264, Follow-up median 1.6 yrs, CHADS2 median 3, double-blind
•
Inclusion: Non-valvular Afib by EKG w/ hx of stroke, TIA, or embolism or with at least a
CHADS2 ≥ 2
•
Randomized to rivaroxaban 20 mg daily or 15 mg daily depending on CrCl vs warfarin
•
Primary outcome: stroke and embolism
•
Safety end point: major and non-major clinically relevant bleeding
13
Non-inferiority margin
1.46
14
15
NOACs and DVT/PE
16
17
December 6, 2009
RE-COVER
•
N = 2564, Follow-up 6 months, double-blind
•
Inclusion: DVT or PE with planned tx for 6 months
•
Exclusion: Symptoms longer than 6 months, PE with HD instability or use of TPA, indication for warfarin, unstable heart disease, high risk of bleeding, transaminases, life expectancy < 6 months, CrCl < 30, pregnancy
•
Randomized to 150 mg dabigatran BID vs warfarin
•
Primary outcome: symptomatic VTE or death 2/2
VTE
18
NIM 2.75
19
20
21
December 2010
EINSTEIN-DVT
•
N = 3449, most tx for 6 months, open-label
•
Inclusion: DVT w/o PE
•
Exclusion: CrCl <30, liver disease, active bleeding or high risk for bleeding, HTN, contraindication to anticoagulation, or received
UFH/LMWH for > 48 hrs
•
Randomized to rivaroxaban at 15 mg BID for 3 weeks then 20 mg daily for 3, 6, or 12 months vs warfarin
•
Primary outcome: symptomatic recurrent VTE
22
NIM 2.0
23
24
NOACs and Overall
Risks
25
26
Fatal bleeding: 1 fewer death per 1000 pts
GI bleeding: 1 increased bleed per 1000 pts
27
Summary
•
Non-inferior for prevention of stroke/embolism in
Afib
•
Non-inferior for treatment of DVT/PE
•
NNT for clinical benefit are large
•
Probable reduced hemorrhagic stroke rate
•
Reduced rate of fatal bleeding events
•
Increased incidence of GI bleeds
•
Perhaps increased incidence of MIs with dabigatran
•
Cost of drug/year $3000
28