The Wheel of Misfortune - European Heart Journal
advertisement
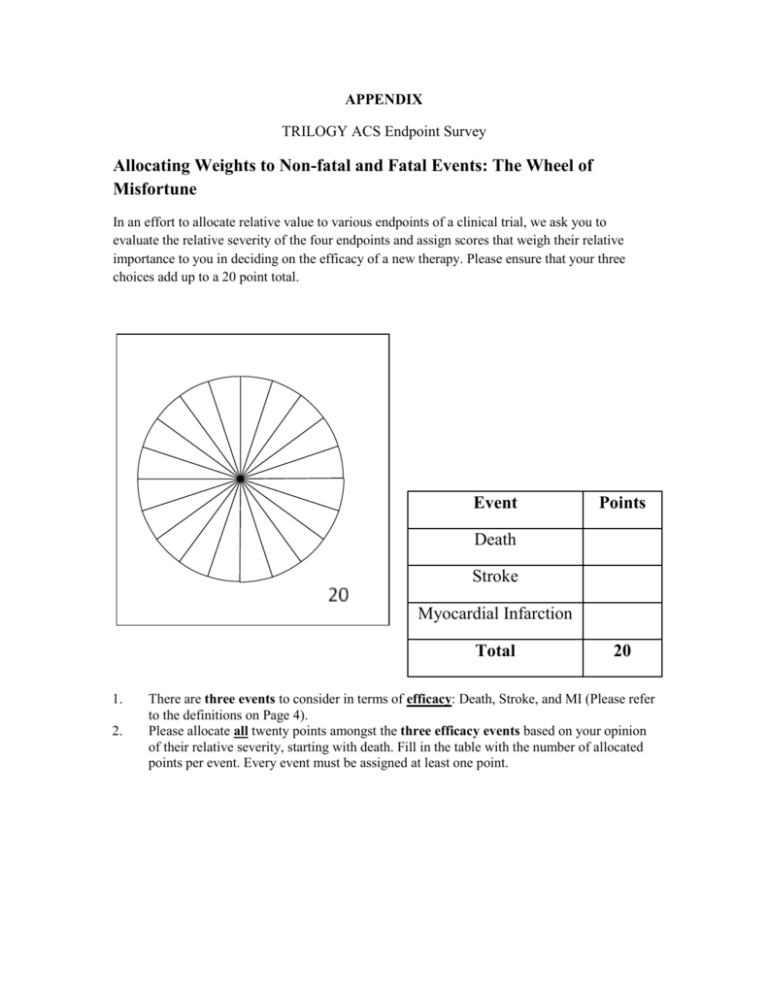
APPENDIX TRILOGY ACS Endpoint Survey Allocating Weights to Non-fatal and Fatal Events: The Wheel of Misfortune In an effort to allocate relative value to various endpoints of a clinical trial, we ask you to evaluate the relative severity of the four endpoints and assign scores that weigh their relative importance to you in deciding on the efficacy of a new therapy. Please ensure that your three choices add up to a 20 point total. Event Points Death Stroke Myocardial Infarction Total 1. 2. 20 There are three events to consider in terms of efficacy: Death, Stroke, and MI (Please refer to the definitions on Page 4). Please allocate all twenty points amongst the three efficacy events based on your opinion of their relative severity, starting with death. Fill in the table with the number of allocated points per event. Every event must be assigned at least one point. Part 2 From: ____________________ Refinement of the Efficacy: Safety Trade-off 1. Please indicate the maximum number of increased bleeding events [i.e. either severe or severe and life threatening] you would accept to achieve a 20% reduction in MI events i.e. 15 per 1000 patients. 20% relative reduction of MI’s Severe/Lifethreatening bleeding Baseline rate 3% Severe bleeding Baseline rate 3% (30 patients/ 1000) (30 patients/ 1000) Type of MI Large 15 per 1000pts ___ per 1000pts ___ per 1000pts Moderate 15 per 1000pts ___ per 1000pts ___ per 1000pts Peri-procedural 15 per 1000pts ___ per 1000pts ___ per 1000pts 2. Please indicate the maximum number of bleeding events you would accept to achieve a 20% reduction in stroke events. 20% reduction in stroke Severe/Lifethreatening bleeding Severe bleeding Baseline rate 3% Baseline rate 3% (30 patients/ 1000) (30 patients/ 1000) Type of Stroke Disabling 3 per 1000pts ___ per 1000pts ___ per 1000pts Non-disabling 3 per 1000pts ___ per 1000pts ___ per 1000pts TIA 3 per 1000pts ___ per 1000pts ___ per 1000pts