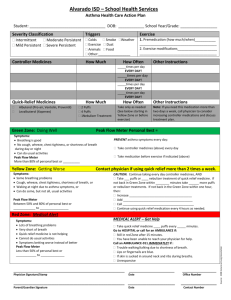
Primary care prescribing - NHS Lothian Respiratory Managed
advertisement

Respiratory Prescribing Maureen Reid and Katie Johnston Primary Care Pharmacists Edinburgh CHP Wednesday 26th November 2014 Respiratory medication (Diagnosis, Drug, Dose) Diagnosis - determines treatment. Treatment – usually includes medication but all medicines have the potential to cause harm as well as provide benefit. Respiratory medicines can have more than one licensed indication. Use LJF and eLJF. Medication needs to be used correctly to be effective- Compliance and concordance essential . Graph showing each Health Board / September 2012 to August 2013 / 03 Respiratory Cost per patient Quality and good practice are related Quality is never an accident; it is always the result of high intention, sincere effort, intelligent direction and skillful execution; it represents the wise choice of many alternatives.” ~William A. Foster “Quality begins on the inside… then works its way out.” ~Bob Moawad “The definition of insanity is doing the same thing over and over again and expecting different outcomes.” – Einstein Scottish Therapeutic Utility Lothian Joint Formulary Medicines and devices can have more than one licensed use and this can be at different doses. Seretide® Evohalers® 50,125,250 Seretide® 250 Accuhaler ® Asthma maintenance – 1-2 puffs BD : for maintenance and reliever I puff BD, for relief of symptoms, 1 puff as needed (max 8 puffs) COPD – Two puffs BD Symbicort ® – 400/12 licensed for asthma and COPD – One puff BD Fostair ® only licensed for asthma – One puff BD Seretide® 500 Accuhaler ® only licensed for asthma – Two puffs BD Asthma (maintenance only) – 1 puff BD reduced to 1 puff once daily if control maintained. COPD – 1 puff BD Symbicort ® – 200/6 Asthma (maintenance and reliever) – 2 puffs in 1-2 divided doses, increased to 2 puffs BD: for reliever of symptoms (SMART regimen) – 1 puff as needed up to 6 puffs at a time, max 8 puffs daily. (12 puffs for limited time) COPD – 2 puffs BD Recent LJF COPD changes COPD (choice determined by inhaler technique) Meter Dosed Inhaler - Fostair ® (beclometasone plus formoterol) Dry powder - Relvar Ellipta ® (fluticasone furoate plus vilanterol) LJF COPD changes SMC restriction - in patients with severe COPD (FEV1 <50% predicted normal). No planned switch as the potency of the steroids in the components is different to what has been used before. Start all new patients on LJF products. Corticosteroids are not licensed on their own in COPD. Both have set doses for COPD: Relvar Ellipta 92mcg/22mcg (low strength) ONE inhalation ONCE a day Fostair TWO puffs TWICE a day The colour of Relvar Ellipta is currently blue but this is due to change to yellow. All new inhalers have a short expiry once opened. Relvar is 6 weeks. Fostair is 5 months once dispensed and out of the fridge. From the Respiratory Prescribing Strategy Figure 10: Prescribing of combination inhalers in children Types of Inhaler Device? Think of inhalers in 2 categories: Aerosol: Liquid medication or Dry powder preparation Effectiveness of the inhaler depends on: patient effort patient technique Inhaler devices These are the main ones! Metered Dose Inhaler (MDI) Easyhaler® Accuhaler ® Clickhaler Turbohaler ® Genuair ® Ellipta ® ® Breezhaler Easi-breathe ® Handihaler ® Respimat ® Novolizer ® Autohaler ® NEXThaler ® Others Integra ® Spacehaler ® Syncroner inhaler ® Easyhaler® device LJF first choice for dry powder Available for Salbutamol, beclomethasone, budesonide, formoterol. eLJF updated so Salamol® Easybreath no longer first choice. Change all Salbutamol dry powder to Easyhaler®. National Therapeutic Indicators High Strength Steroid inhalers (not Fostair®) as a percentage of all Steroid inhalers (items) Report Period: July 2014 to September 2014 Lower Quartile Mid Quartile Upper Quartile Scotland Baseline (January 2014 to March 2014) 30.49% 38.03% 44.96% NHS LOTHIAN for period of report 35.18% 42.43% 49.47% What is considered “high dose” ICS? <800mcg/day BDP Eq (FP 400mcg, QVAR 400mcg) unlikely to cause any detrimental effects apart from local Marked HPA suppression at dose above 1500mcg/day (FP 750mcg) Lipworth et al. Ach Intern Med 1999; 159;941-55 Considerable inter-individual susceptibility Also consider: Standard doses of ICS used in conjunction with other steroids (such as oral/nasal steroids) Use of ICS with concomitant medicines that inhibit their metabolism (cytochrome P450 inhibiting drugs: e.g. HIV protease inhibitors) Minimising the steroid load with inhaled corticosteroids. LPB 57 Sept 2012 Dose of ICS ? 1. Masoli M et al. Thorax 2004; 59:16-20 2. Holt S et al. BMJ 2001: 323:253-256 Top Top of clinical dose response curve: 500 mcg/day FP1,2 = ~400 mcg/day Qvar = 800 mcg/day Clenil = 800 mcg/day Bud 90% effect achieved at doses: 200 mcg/day FP1,2 = 200 mcg/day Qvar = 400 mcg/day Clenil = 400 mcg/day Bud Starting a combination steroid inhaler in COPD Scottish Medicines Consortium (SMC) states these preparations can be used in the treatment of adults with severe COPD (FEV1 <50% predicted normal). Should be reviewed after 3 months – not everyone has a benefit. COPD review Measure treatment effectiveness by; Improvement in symptoms Increase in activies of daily living Improvement in exercise tolerance Questions to assessment response to therapy has your treatment made any difference to you? Is your breathing any easier? Can you do things now that you could not do before? Can you do things faster than before? Can you do the same things now but with less breathlessness? The cost of Prescribing ..... ...... the Harms of high dose ICS Pneumonia The cost of Prescribing ..... ...... the Harms of high dose ICS Prolonged use of high doses of ICS carries a risk of systemic side-effects, including adrenal suppression or crisis, growth retardation in children and adolescents, decrease in bone mineral density, cataract and glaucoma In addition, a range of psychological or behavioural effects may also occur. These include psychomotor hyperactivity, sleep disorders, anxiety, depression, and aggression (particularly in children) Other side-effects: Diabetes* (risk of onset over 5.5 yrs – RR 1.34, High dose RR 1.64 – NNH 21) * Suissa S, et al. Am J Med 2010;123:1001–6 Patient Adherence Medication adherence by patients is generally poor, with reports citing adherence rates to various treatment regimens of approximately 50%. 25% of patients have asthma adherence rates estimated at 30% or less Non-adherence is thought to contribute to 18% to 48% of asthma deaths Optimise lung deposition: Inhaler technique Overall, up to 90% of patients show incorrect inhaler technique in clinical studies Patients’ inhaler technique can be significantly improved by brief instruction given by trained HCP However, 25% of patients have never received verbal inhaler instruction. Only an estimated 11% of patients receive follow-up assessment and education Ask “Can you show me how you use your inhaler?” 75% of patients using an inhaler for on average 2-3 years reported they were using their inhaler correctly but on checking only 10% demonstrated correct technique (Basheti IA et al (2008) Why is it important? Essential to ensure patients are receiving inhaled medications May prevent basic treatment failure Correct device for patient may improve symptom management Prevent inappropriate escalation of treatment – reduce costs Reduce risk of adverse effects We wouldn’t let a patient put a tablet in their ear, what's the difference of letting them have bad inhaler technique? Asthma - 3 key things to remember 1. 2. 3. Follow guidelines – add in a LABA before increasing steroid Check inhaler technique, and compliance before any alteration in therapy (device /drug/dose). Review regularly, using ACT and step down (need to be familiar with equivalent doses) COPD- 3 things to remember 1. 2. 3. Review new treatment after 3 months – check compliance and if no benefit stop Dual diagnosis – treat as asthma Be familiar with licensed devices and doses for COPD Summary Asthma and COPD are different conditions and are treated differently Licensed inhaler products and doses vary depending on the condition Always check inhaler technique Always check compliance Use LJF Use Scottish Therapeutic Utility (STU) to review patients who don’t order their inhalers