Chemistry Ch. 5
advertisement

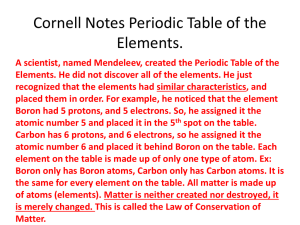
Chemistry Ch. 5 Periodic Law 5-1 History of the Periodic Table 1860-Cannizzaro discovered a method for accurately measuring Relative Masses, which led chemists to agree on standard values for atomic masses of elements This led to a search for a relationship between mass and other properties of the then 60 discovered elements Mendeleev Mendeleev wanted to organize the elements according to their properties so he wrote each element on a card, along with its mass and its chemical and physical properties and looked for trends/patterns. He noticed that when he put them in order of increasing atomic mass, certain similarities appeared in their chemical properties at regular intervals (periodic). 1869- 1st periodic table pg. 124 Placed in vertical groups of increasing atomic mass so that the horizontal rows resembled each other chemically Mendeleev’s First Periodic Table This table left empty places in the table where he felt something should be Mendeleev is given credit for the periodic law (We’ll discuss periodic law in a bit) 1. 2. Two questions remained regarding Mendeleev’s table: Why could most of the elements be arranged in the order of increasing atomic mass but a few could not? What was the reason for chemical periodicity? 1911-(40 years later and after discovering all the subatomic particles) Moseley noticed that the elements fit better with patterns when he placed them in order of increasing number of protons (atomic number). Periodic Law-The physical and chemical properties of the elements are periodic functions of their atomic numbers. Modern Periodic Table Chemists have discovered new elements to fill the spaces and even synthesized new ones Periodic Table-an arrangement of the elements in order of their atomic numbers so that elements with similar properties fall in the same column or group Noble Gases: Group 18-chemically unreactive Lanthanides: 14 elements with atomic numbers from 58 to 71 with similar physical and chemical properties Actinides: 14 elements with atomic numbers from 90 to 103 These last two groups belong in periods 6-7 but are set off below the main table to save space Periodicity can be seen in any group of the periodic table Fig. 5-4 The differences in each element of Group 1 and Group 18 are the same The elements in Group 2 and Groups 1317 show a similar pattern. 5-2 Electron Configuration and the Periodic Table Chemical properties are a result of the electron configuration of an element Ex: Noble Gases-the highest energy level is occupied, making the noble gases stable and unreactive -highest configuration level governs the properties of the element Groups-Vertical column of elements, show related elements with similar properties Periods-Horizontal row of elements, the length depends on the sublevel being filled Principal Quantum number of the highest occupied energy level tells us the period the element is in (# protons) Atomic No. 14 Groups (down) Si Element Symbol 28.086 Atomic Mass Silicon Element Name Atoms are Neutral: (#protons = # electrons) How many protons? 14 Periods (across) How many electrons? 14 Sublevel Blocks Fig. 5-5 Based on the electron configurations of the elements, the periodic table can be divided into four sublevel blocks Pg. 129 Sublevel Periodic Table Groups Alkali metals-Group #1 Li-Fr -silvery appearance and are soft enough to cut with a knife -so reactive, they are not found in nature and have to be stored in kerosene because they react violently with air or moisture. -melting point decreases down the group -have 1 electron in outer energy level Groups or Family Names 18) NOBLE GASES Inner earth metals 17) HALOGENS 1) ALKALI METALS 2) ALKALINE METALS TRANSITION METALS Alkaline-earth metals- Group #2 Be-Ra -harder, denser, and stronger than alkali metals -have higher melting points than Group #1 -less reactive than Group #1, but still too reactive to be found in nature -have 2 electrons in outer energy level Hydrogen and Helium Hydrogen does not share the same properties as Group #1; does not share properties with any other group in the table Helium does not behave like Group #2 but like Group #18 so it is part of the Noble Gases (Group 18). Transition Elements D-block elements that are metals with typical metallic properties -good conductors of electricity and a high luster -less reactive than alkali metals or alkaline earth metals -do not easily form compounds, exist as free elements Main Group Elements P-block elements together with the s-block elements -properties vary greatly -number of electrons equals the group #10 Halogens Group 17: F-At -most reactive nonmetals -react vigorously with metals to form salts 7 electrons in the outer energy level Lanthanides and Actinides F-block elements Lanthanides-shiny metals similar reactivity to Group 2 elements Actinides-all radioactive Metals Properties of metals and nonmetals Metals Luster (shiny silvergray, gold, copper) Nonmetals Dull (all different colors) Malleable (hammered) Brittle Ductile (drawn into thin wire) Not ductile High Density & High Melting Points Low Density & Low Melting Points Conductors of heat & Poor Conductors of electricity heat & electricity Lose electrons Gain electrons Metalloids have both metallic and nonmetallic characteristics –along the staircase. Boron, Silicon, Germanium, Arsenic, Antimony, Tellurium, Astatine, Used in electronics 5-3 Electron Configuration and Periodic Properties Atomic Radii-as one-half the distance between the nuclei of identical atoms that are bonded together Period-smaller atomic radii across a period due to increasing the positive charge of the nucleus Group-increase down a group due to increase in size of the atom Ionization Energy Ion-atom(s) that has a positive or negative charge due to gain/loss of an electron Ionization-process that results in the formation of an ion Ionization energy-the energy required to remove one electron from a neutral atom of an element Ionization Energy Period-increase across a period, generally Group-decrease down the group, generally Table 5-3 Highlighted energy reflects the energy needed to remove an electron from the noble gas configuration or the full energy level Electron Affinity The energy change that occurs when an electron is acquired by a neutral atom Most atoms release energy when they acquire an electron Energy released represented by a negative number ex: Group 17 Energy absorbed represented by a positive number ex: Group 1 or Group 18 This book uses negative numbers to represent the energy released Period-generally, become more negative across each period (increase-release more energy as we go across because we acquire more electrons ) Group-not as regular, electrons add with greater difficulty down a group (decreaserelease less energy going down because acquire fewer electrons) Ionic Radii Cation-positive ion Anion-negative ion Period-metals at the left tend to form cations and the nonmetals tend to form anions Cationic radii decrease across a period due to shrinking electron cloud Anionic radii decrease across a period Group-gradual increase of ionic radii down a group Valence Electrons The electrons available to be lost, gained, or shared in the formation of chemical compounds (in the outer shell) Groups 1 & 2 have 1 & 2 electrons, respectively Groups 13-18 have valence electrons equal to group # - 10 Electronegativity A measure of the ability of an atom in a chemical compound to attract electrons Period-increase across each period, with some exceptions Group-decrease down a group or stay about the same Highest electronegative element=Fluorine Electronegativity numbers Trends