Section 6.1
advertisement

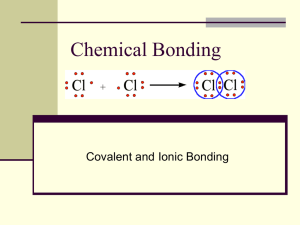
Happy Valentine’s Day on Friday! Wednesday, Feb. 12th: “A” Day Thursday, Feb. 13th: “A” Day Agenda Chapter 5 tests/Chapter 5 Summary Begin Chapter 6: “Covalent Compounds” Sec. 6.1: “Covalent Bonds” Covalent bond, molecular orbital, bond length, bond energy, non-polar covalent bond, polar covalent bond, dipole Homework: Section 6.1 review, pg. 198: #1-14 Concept Review: “Covalent Bonds” Chapter 5 Tests Class 2A 4A 1B 3B Average Score (out of 65) 54.63 52.56 54.29 58.56 Average Percentage 84.05% 80.86% 83.52% 90.09% Sec. 6.1: “Covalent Bonds” When an ionic bond forms, electrons are rearranged and are transferred from one atom to another to form charged ions. In another kind of bond, the neutral atoms share electrons. Sharing Electrons The simplest example of sharing electrons is found in diatomic molecules, such as hydrogen, H2. Since the 2 atoms are the same, neither atom will remove the electron from the other, they will share them equally. The result is that the H2 molecule is more stable than either hydrogen atom alone. By sharing their 1 electron, each hydrogen atom obtains an electron configuration like the noble gas helium. Formation of a Covalent Bond A molecular orbital is made when 2 atomic orbitals overlap. Sharing Electrons Covalent bond: a bond formed when atoms share one or more pairs of electrons. The shared electrons move within a space called a molecular orbital. Molecular orbital: the region of high probability that is occupied by an individual electron as it travels with a wavelike motion in the threedimensional space around one of two or more associated nuclei. In plain English: a molecular orbital is the space that the shared electrons move around in. Energy Is Released When Atoms Form a Covalent Bond 2 H atoms When 2 bonded hydrogen atoms are at their lowest potential energy, the distance between them is 75 pm. (1 pm = 1 X 10-12 m) Potential Energy Determines Bond Length Bond length: the distance between two bonded atoms at their minimum potential energy; the average distance between the nuclei of two bonded atoms. The two nuclei in a covalent bond vibrate back and forth and the distance between them is constantly changing. That’s why the bond length is the average distance between the two nuclei. Bonds Vary in Strength Bond Energy: the energy required to break the bonds in 1 mole of a chemical compound At a bond length of 75 pm, the potential energy of H2 is –436 kJ/mol. That means that 436 kJ of energy must be supplied to break the bonds in 1 mol of H2 molecules. Bonds that have higher bond energies (stronger bonds) have shorter bond lengths. (shorter bond length= stronger bond) Electronegativity and Covalent Bonding Remember: electronegativity is how much an atom in a molecule attracts electrons. Electronegativity values are a useful tool to predict what kind of bond will form between 2 atoms. Polar vs. Non-Polar Covalent Bonds Depending on how the electrons are shared, covalent bonds can be either polar or non-polar. Polar means: “opposite in character” Think of the North pole and the South pole Atoms Can Share Electrons 2 Different Ways 1. Equally 2. Unequally Atoms Can Share Electrons Equally When the electronegativity values of two bonding atoms are similar, bonding electrons are shared equally and a non-polar covalent bond is formed. Non-polar covalent bond: a covalent bond in which the bonding electrons are equally attracted to both bonded atoms. Examples: Cl2 O2 H2 Atoms Can Share Electrons Unequally When the electronegativity values of two bonding atoms are different, bonding electrons are shared unequally and a polar covalent bond is formed. The atom with the higher electronegativity “hogs” the electrons away from the other atom. Polar covalent bond: a covalent bond in which a shared pair of electrons is held more closely by one of the atoms. Predicting Bond Character from Electronegativity Differences Use the table of electronegativities on pg. 194. Find the difference in electronegativity values between the bonded atoms to predict bond character. Predicting Bond Character Examples Predict what type of bond would form between the following atoms: 1. H and Br Electrongegativity of Br = 3.0 Electronegativity of H = 2.2 Electronegativity difference = 0.8 polar covalent Predicting Bond Character Examples Predict what type of bond would form between the following atoms: 2. Cl and Cl Electrongegativity of Cl = 3.2 Electronegativity of Cl = 3.2 Electronegativity difference = 0.0 (same atom will share electrons equally) non-polar covalent Predicting Bond Character Examples Predict what type of bond would form between the following atoms: 3. Li and Cl Electrongegativity of Cl = 3.2 Electronegativity of Li = 1.0 Electronegativity difference = 2.2 ionic Polar Molecules Have Positive and Negative Ends In a polar covalent bond, the ends of the bond have opposite partial charges because one of the atoms is “hogging” the electrons. Dipole: a molecule or part of a molecule that contains both positively and negatively charged regions. In a polar covalent bond, the shared pair of electrons are more likely to be found near the more electronegative atom. Polar Molecules Have Positive and Negative Ends The Greek symbol (delta) means partial. + is used to show a partial positive charge – is used to show a partial negative charge Example: H+ F– is polar covalent Because the F atom has a partial negative charge, the electron pair is more likely to be found closer to the fluorine atom. Polarity Is Related to Bond Strength In general, the greater the electronegativity difference, the greater the polarity and the stronger the bond. Polarity Is Related to Bond Strength Think of it this way: You know that ionic bonds are stronger than covalent bonds. The greater the difference in electronegativity, the closer the covalent bond comes to being like an ionic bond. Electronegativity and Bond Types Differences in electronegativity values provide one model that can tell you which type of bond two atoms will form. Another general rule states: An ionic bond forms between a metal and a non-metal. A covalent bond forms between two non-metals. Properties of Substances Depend on Bond Type The type of bond that forms (metallic, ionic, or covalent) determines the physical and chemical properties of the substance. Metallic bonds: good conductors in solid state because electrons are free to move around. Ionic bonds: strong attraction between ions makes them solids with high melting/boiling points. Covalent bonds: weaker attraction between molecules makes them gases/liquids at room temperature or solids with low melting/boiling points. Homework Section 6.1 review, pg. 198: #1-14 Concept Review: “Covalent Bonds” Concept reviews MUST be turned back in before you leave today! Next time: Sec. 6.1 quiz: “Covalent Bonds” Lab write-up AND lab Dress appropriately please!