Covalent Molecules, Networks & Layers
advertisement

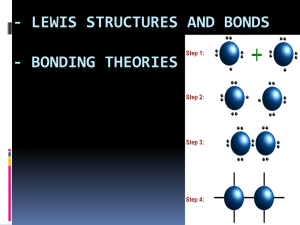
Chapter 7 – Covalent Molecules, Networks and Lattices Term 2, Week 1, Lesson 1 Three Different Forms of Carbon • Charcoal, Diamond and Graphite all consist of the non-metal carbon. • They all have the chemical composition, C. • Charcoal, Diamond and Graphite are different physical forms of carbon. • These are called allotropes of carbon. • Their physical properties are very different. Diamond • Diamond was found in India thousands of year ago. • It is such a prized jewel because when cut and polished it sparkles with brilliance. • It is the hardest naturally occurring substance. Charcoal • Charcoal is used to fuel fires for many years. • It is also used for drawing. • Charcoal is a black powdery substance. Graphite • Graphite is used: – In ‘lead’ pencils – As an electrode in some torch cells (batteries), which have a rod of graphite in the centre – As a dry lubricant in place of grease in machinery that runs at high temperatures – As an additive to make rubber and plastic materials more flexible – As fibres in recently developed sports equipment. Graphite cont… • The properties that make graphite so useful are its ability to conduct electricity in solid state, its soft, greasy nature and high melting temperature. • It is a grey opaque solid. Carbon Bonds • A diamond consists of carbon atoms bonded very strongly to other carbon atoms in a 3D lattice. • Diamond is hard and crystalline and has a very high melting temperature. • Graphite is very hard in 2 dimensions but slippery and soft in the other direction. • It consists of layers of carbon atoms. • Within each layer the carbon atoms are very strongly bonded to the other 3 carbons atoms but between layers there is very weak bonding. • The structure of carbon is currently the subject of research. • It appears that charcoal may be made up of fragments of buckyballs, which are particles shaped roughly like a soccer ball and made up of groups of 60 carbon atoms. Compounds of Carbon • Carbon readily forms many compounds with other non-metals and many of these compounds consist of very small molecules which are gases at room temperature. Carbon Dioxide • Carbon dioxide consists of a carbon atom bonded to 2 oxygen atoms. • The CO2 molecule is small with very strong bonds holding the carbon and oxygen atoms together but very weak forces between the molecules. • This means that the molecules are easy to separate and so dry ice sublimes (goes from solid to gas with no liquid phase). Carbon & Bonding • The type of bonding between the carbon atoms in diamond and graphite and between carbon and oxygen in carbon dioxide is all the same type. • They are all covalently bonded, this means that electrons are shared to produce full outer shells. Covalent Bonds • Atoms in a covalent bond are atoms of nonmetallic elements. • Some other covalently bonded substances, such as diamond and graphite, exist as covalent lattices, in which a huge number of atoms are joined together. • Most substances that exist as small covalent molecules are liquids or gases at room temperature. Covalent Bonds etc… • There are only weak forces of attraction between the molecules. • These forces are relatively easy to overcome and so the melting or boiling temperature of these substances tend to be low. • All the substances that exist as covalent lattices are solids at room temperature because all the atoms in the lattice are held to one another by strong covalent bonds. Comparing Diamond, Graphite and Charcoal Bonding Models • When a covalent molecular substance melts, the molecules separate for each other yet the atoms within the molecules remain bound to one another. • This means that the bonding model for molecular substances must describe two kinds of forces: – The strong forces of attraction which holds atoms together within molecules; – The weak force of attractions between molecules. Covalent Bonding within Molecules • When non-metals combine electrons are shared so that each atom has eight electrons in its outer shell. • In the case of hydrogen, two outer electrons is a stable configuration. • Molecules formed in this way are more stable than the separate atoms. Small Molecules with Single Covalent Bonds • Hydrogen (H2) – The simplest molecule is that formed when hydrogen atoms bond. – The melting temperature of solid hydrogen is -259 degrees, indicating that the forces between hydrogen molecules are extremely weak. – In contrast, an extremely large amount of energy is required to separate the two hydrogen atoms of a H2 molecule. Hydrogen cont… – When two hydrogen atoms form a hydrogen molecule, two electrons, one from each H, are shared between the two atoms. – These two electrons spend their time between the two nuclei and are considered to be localised. – This region is where the –ve charged electrons will have the greatest attraction to both +ve charged nuclei. – The strong force of attraction involving a shared pair of electrons is called a single covalent bond Small Molecules cont – Chlorine (Cl2) • A chlorine atoms needs one more electron to achieve a stable outer shell. • The two atoms in a chlorine molecule are joined by a single covalent bond. • Of all of the outer shell electrons, only two are shared. • The other electrons are distributed around the two atoms. Chlorine (Cl2) cont… • The electrons that are shared are known as the bonding electrons. • The outer shell electrons not shared are called the non-bonding electrons. • Pairs of non-bonding electrons are called lone pairs. • This means that chlorine has one bonding pair and three lone pairs. • Chlorine needs to share one electron, so we say that it has a covalency of 1. • The covalency of an atom is generally the number of electrons it shares when covalently bonded. Chlorine (Cl2) cont… • However, this cannot be assumed for all situations. • There are a number of different compounds in which atoms do not achieve an octet of electrons or exhibit different covalencies in different molecules for various reasons. • For example, sulfur forms a number of stable oxides, SO2 and SO3, each with sulfur exhibiting a different covalency. Chlorine (Cl2) cont… Charge Cloud Diagrams • Are more realistic ‘pictures’ of hydrogen and chlorine molecules. • They attempt to represent the fact that the electrons within the molecules are not stationary but are continuously moving. • This gives the appearance of negative charge clouds in the molecules. Charge Cloud Diagrams cont… Small Molecules cont – Hydrogen Chloride (HCl) • • • • • H electron configuration is 1 Needs one electron for stable shell So forms one covalent bond by sharing 1 electron Chlorine electron configuration is 2,8,7 So forms one covalent bond by sharing 1 electron, leaving six non-bonding electrons (three non-bonding lone pairs) • In a hydrogen chloride molecule, each atom contributes one electron to form a shared pair of electrons. • The hydrogen atom can be considered to have a stable outer shell of two electrons and the chlorine a stable outer shell of eight electrons. Hydrogen Chloride (HCl) A representation of a hydrogen chloride molecule, showing the outer-shell electrons. A charge cloud diagram of hydrogen chloride. Your Task • Look over pages 115-118 • Come up with your own summaries for – Molecules with more than two atoms – Some simple hydrocarbons – Some small molecules with double or triple bonds – Representing molecules Week 1, Lesson 2 Shapes of Molecules • The shape of a molecule describes the way in which the atoms are arranged. • For a simple molecule such as hydrogen, or hydrogen chloride the shape is linear. • The two atoms must be in a straight line. • For molecules with more than two atoms the situation is not so simple. Shapes of Molecules cont… • Consider a molecule of methane. • The central carbon is surrounded by four separate pairs of electrons in its outer shell. • Each pair represents a single covalent bond between the carbon atom and a hydrogen atom. • These electrons pairs are negatively charged and so repel each other. Shapes of Molecules cont… • Molecules are most stable when the electron pairs, both bonding and non-bonding, are as far apart as possible. • The maximum separation possible for the four pairs of valence electrons is described as tetrahedral. Tetrahedral Formation Water’s Formation • Looking at the valence structure for water, the oxygen atom has four separate pairs of electrons around it. • These four pairs adopt the same arrangement as methane. • But, when describing the shape of a water molecule, the most important thing is the position of the atoms. • A water molecule may be described as angular or vshaped. • Although the lone pairs help to determine the shape of the molecule, they are not included in the description. • Shape describes the positions of the atoms only. Ammonia • Is a different shape from that of methane. • This is because one point of the tetrahedron contains a lone pair of electrons. • The four separate electron pairs around the nitrogen atom assume a tetrahedral orientation, however, only three points of the tetrahedron are occupied by hydrogen atoms. • Therefore, the molecular shape is a triangular pyramid. Hydrogen Fluoride • The orientation of the four pairs of electrons (three non bonding lone pairs and one bonding pair) around the fluorine atom is also tetrahedral. • Because there are only two atoms in the molecule, the shape of the molecule is linear. Molecule Shapes VSPER Model • The valence shell electron pair repulsion model states that electron pairs in the outer shell of an atom in a molecule will repel one another due to their negative charge and assume positions as far apart as possible, while remaining attached to the atom. VSPER Model cont… • In all four molecules, CH4, NH3, H2O and HF, the central atom has four pairs of electrons in its outer shell. • So in each case, the electron pair geometry is tetrahedral which the molecular shape is determined by the positions of the atoms. • The VSPER model applies equally to molecules where the central atom has either less or more than four electron pairs around it. • Some examples of simple molecules where the central atom does not obey the octet rule are given on the next slide. Exceptions… (a) The valence structure of BeH2 is linear because the central atom, Be, is surrounded by two pairs of electrons only. (b) BF3 has the shape of a planar triangle. B has three pairs of valence electrons which are arranged at 120° from one another to achieve maximum stability. (c) In SF6, the six F atoms are arranged at 90° from one another, the maximum distance possible for six electron pairs. Its shape is octahedral. Carbon Dioxide (CO2) • Carbon has an electron configuration of 2,4 so it needs four electrons for a stable outer shell. • Oxygen has an electron configuration of 2,6 and so need to electrons for a stable outer shell. • The central atom, carbon, forms a double bond with each oxygen. • The four electron pairs are concentrated in two regions of negative charge only, each one consisting on a double bond. • These two regions repel and so assume positions as far from one another as possible. • That is, on opposite sides of the carbon atom. • See figure 7.20, page 121 Week 1, Lesson 3 Forces between Molecules • If you rub a plastic or glass rod with a piece of fur or nylon and hold the rod next to a thin stream of water from a tap, you will see how the water is attracted to the charged rod. • This suggests that the water molecule has some charge although the model of a molecule does not show this. • Since some molecules other than water also behave as though they have some charge the model will need to be modified. Polarised Bonds • When two different non-metals form a covalent bond, one atom usually attracts the bonding electrons more strongly. • In HCl, the chlorine atom attracts the bonding electrons more strongly than the hydrogen atom. • Chlorine is said to have a greater electronegativity than hydrogen. • This means that the bonding electrons spend more time closer to the chlorine atoms. • The chlorine end of the molecule becomes slightly negative and the hydrogen atom is slightly positive. Polarised Bonds cont… • The chlorine only has a partial charge, not a whole negative charge, and hydrogen a partial positive charge. • The covalent bond in the hydrogen chloride molecule is said to be polarised and the molecule is a polar molecule. • The molecule is said to be a dipole, because it has two charged ends, or poles. • In solid hydrogen chloride, the molecules will be arranged so that the oppositely charged ends of the dipoles are next to each other. • It is these forces that hold them together. • This type of attraction is called dipole-dipole attraction. Polarised Bonds cont… Intermolecular Forces and Melting • Dipole-dipole attraction forces are strong enough to hold hydrogen chloride molecules together in a solid lattice at low temperatures. • These forces are much weaker than the covalent bonds within the molecule. • When this sort of bond is heated to its melting temperature, the molecules gain sufficient energy to overcome some of the forces of attraction and the lattice breaks to form a liquid, however, each molecule remains intact because of the strong covalent bonds. • The dipole-dipole forces have not totally disappeared, they are still present and hold the molecules in a liquid state. Identifying Polar Molecules • Compare the structural formulas below… Formaldehyde has the polar bonds arranged in such a way that each end has an opposing charge. The oxygen end has a negative charge, while the hydrogen end has a positive charge. The molecule is therefore a dipole. The carbon dioxide, however is symmetrical. Both ends have a negative charge, with the positive in the centre. Both the negative ends are equal and therefore is not a dipole. Identifying Polar Molecules cont… • The molecule tetrafluormethane (CF4) is also non-polar. Although each of the covalent C-F bonds are polarised, the molecule itself does not have a negative and a positive side. • So two conditions must apply if a molecule is said to be dipole: – It must have polar bonds – The partial charges must be distributed asymmetrically across the molecule. – Polar molecules will attract each other with the negative side of one molecule attracting the positive side of another. This weak dipole-dipole attraction helps hold the molecules together in a polar covalent molecular solid. Hydrogen Bonding • Is the most significant kind of dipole-dipole attraction. • Hydrogen bonding occurs between molecule in which hydrogen is bonded to N, O or F. • It occurs because: – N, O and F are highly electronegative atoms – The bonds between hydrogen atoms and each of these other atoms are highly polarised – There is a relatively large partial positive charge on the hydrogen atom when it is bonded to any of these atoms – N, O and F atoms each have at least one lone pair of electrons when they form molecules. Hydrogen Bonding cont… • The H-bonding between the partially positive hydrogen atom of one molecule and the lone pair on a N, O or F atom of a neighbouring molecule is much stronger than other cases of dipole-dipole bonding. • Hydrogen bonding in not another type of bonding such as ionic, metallic and covalent bonding. • Hydrogen bonding is an intermolecular force and only occurs from one covalent molecule to another under the conditions mentioned. • Hydrogen bonds are much weaker than ionic, metallic or covalent bonds. • They are more significant then other forms of dipole-dipole attraction. Hydrogen Bonding cont… Weakest Bonding Forces • The weakest bonding force operates in all substances. • It is most important when it is the only force between particles. • It arises as a result of the constant movement of electrons in atoms and molecules. • At one instant, it is very unlikely that all electrons in the atom or molecule are distributed evenly around the nucleus. It’s more likely that at one point there are more electrons at one ‘side’ than the other. Weakest Bonding Forces cont… • This causes the atom or molecule to be momentarily polar and so electrostatically interact with neighbouring atoms or molecules. • Because the electrons are moving so rapidly, the direction of these instantaneous interactions are constantly changing. • These instantaneous dipoles results in a net, but weak force of attraction between all particles. Weakest Bonding Forces & Noble Gases • The effect of this force can be seen if you examine the melting and boiling temperature of the noble gases. • Bonding models are based on the observations that the atoms of noble gases rarely combine with other atoms. • They rarely gain, lose, or share electrons by reacting with other substances. • As elements, the particles present in the solid, liquid and gas states of noble gases are the individual atoms. • The fact that liquid and solid states of these elements can be formed means that there must be forces between the atoms. • The very low values for their melting and boiling temperatures indicate that these forces are extremely weak. Weakest Bonding Forces & Noble Gases cont… • The melting and boiling temperatures of the noble gases increase as the sizes of the atoms increase. • Even though these forces are weak they become more significant as the number of electrons, and size of the atom, increases. • These forces are only considered when no significantly stronger forces exist. • They are known as dispersion forces or van der Waal’s forces and are responsible for holding the molecules together in non-polar molecular substances. • Examples of these substances include molecular oxygen, hydrogen, nitrogen and methane, as well as the noble gases. Weakest Bonding Forces (a) How an instantaneous dipole forms in a chlorine molecule. (b) How an instantaneous dipole in one xenon atom interacts with a neighbouring xenon atom. Week 2, Lesson 1 Covalent Lattices • Diamond and graphite are giant covalent lattices. • Carbon dioxide is a small covalent molecule. • Carbon dioxide has strong bonds between the carbon and two oxygen atoms. However, the bond between molecules is weak. This is why it is a gas at room temperature. • Diamond and graphite form covalent bonds continuously throughout the lattice. • The structure of diamond is known as a network lattice. • The structure of graphite is described as a layer lattice. Covalent Network Lattices • Diamonds are the hardest naturally occurring substance and is also used widely in machinery for drilling, cutting and polishing. • In diamond, each carbon atom is covalently bonded to four other carbons, and the bonding extends through the lattice. • Four bonds are directed away from each carbon atom at the angle of a regular tetrahedron. • The strong covalent bonds are continuous throughout the lattice. • There are no weak links. • See page 129, Figure 7.32 for structure of diamond Graphite: A Covalent Layer Lattice • Graphite is very hard in one direction, but quite slippery and soft in another direction. • It has a layered structure. • The carbon atoms within the layers are held together by covalent bonds. • These bonds within the layer are very strong. • The forces between layers are weak dispersion forces. • This structure is known as a covalent layer lattice. Covalent Layer Lattice cont… • Within a layer, each carbon atom is bonded to three other carbon atoms. • The 4th electron from each carbon is delocalised, it is free to move within the layer. • These electrons allow graphite to conduct electricity. • In graphite, the distance between layers is much greater than the carbon to carbon length within the layer. • See page 129, Figure 7.33 for structure of graphite. Using the Models of Bonding • Covalent molecular, network and layer lattice models of bonding can be used to explain the similarities and differences in properties of non-metal elements and compounds. Melting and Boiling Temperatures • When a substance melts the particles break out of their positions in the lattice and move randomly in the liquid state. • For substances composed of small molecules, the forces between the molecules are weak. • Only a small amount of energy is required to disrupt the intermolecular forces. • Therefore, substances with small molecules have a low melting temperature. Melting and Boiling Temperatures cont… • In network and layer lattices, a large amount of energy is needed to disrupt the lattice. • They have strong covalently bonding throughout the lattice and have high melting temperatures. • At high temperatures, all bonds break and atoms are free to move independently. Electrical Conductivity • An electric current will run through a substance when there are charged particles are free to move. • Covalent molecules have no overall charge. • Because the molecules are neutral and there are no delocalised electrons, covalent molecular substances cannot conduct electricity in solid or molten state. • In network lattices, all electrons are localised, as such there are no free moving electron to carry an electrical current. • In graphite, one electron from each carbon atom is delocalised and is free to move. This allows electricity to be conducted. Hardness and Softness • Network lattices are usually very hard, eg, diamond. They have strong covalent bonds throughout the lattice with all atoms being a fixed positions. • In graphite, the forces between layers are weak and so layers can slide over one another. Graphite appears soft and greasy in one direction. • In molecular solid, the molecules adopt fixed positions and form a regular lattice. The lattice is crystalline and can be hard. For example, ice where the water molecules are held in rigid positions to maximise the possibility of hydrogen bonding. • However, because of the intermolecular forces of attraction being weak, covalent molecular substances are often liquid or gases at room temperature. Chemical Reactivity • It is very hard to disrupt a strong bond. • Substances composed of covalent network lattices like diamond or silica do not react readily. • Graphite, a covalent layer lattice, is also very unreactive. • Small covalent molecules are much more reactive. • This reactivity depends on the strength and stability of the bonds. For example, triple bonded nitrogen is not very reactive, where a double bonded oxygen is more reactive. • The reactivity of small covalent molecules also depends on a number of factors including polarity of bonds and nature of the arrangement of the atoms in the molecule.