Mrs. Wolfe
advertisement

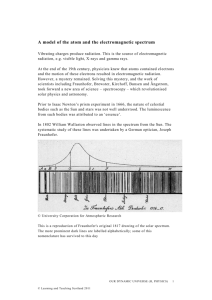
Bohr Models and Lewis Dot Structures Recall that electrons orbit the nucleus in energy levels • Electrons orbit the nucleus in rings. • Layers of Rings – Closest to nucleus • Lower energy level – Farthest from nucleus • Higher energy level • Each ring can hold only a certain number of electrons. Rings hold certain numbers of Electrons • All of the rings together are called the electron cloud. • But separately, each ring has its own “name.” • First Energy Level – Can hold 2 electrons • Second Energy Level – Can hold 8 electrons • Third Energy Level – Can hold 8 electrons • Fourth Energy Level – Can hold 2 electrons Let’s Draw a Picture of an Argon Atom. 18P 14N • You know how many protons, electrons, & neutrons are in an atom of any element. Now, draw it. How many e- are in the … • First Energy Level? • Second? • Third? • Fourth? • This is called a Bohr Model!! Bohr Models • We call this model the Bohr Model, after Neils Bohr who developed it in 1915. BOHR MODEL •It has a circle in the center, which represents the nucleus. We write the number of protons and neutrons in this circle. •The rings surrounding the circle are the energy levels. We draw electrons on the energy levels. Making a Model of an Atom • Draw the nucleus, the energy levels, and fill in the electrons in each energy level. • ALWAYS FILL ENERGY LEVELS BEGINNING WITH THE FIRST ENERGY LEVEL!! Let’s Try a Bohr Model of a Lithium Atom. Did you get it right? If not, here’s a Review: • Figure out how many Protons, Neutrons, & Electrons are in the atom. • Write the number of Protons & Neutrons in the nucleus. Why there? • Fill the energy levels in order beginning with FIRST energy level! (2, 8, 8, 2) • It’s OK to have empty energy levels on the outside. One More Practice Problem… Draw a Bohr model of Oxygen. Did you get it right? Term to Know • Valence Electron – number of electrons in the outer energy level. • What is the Valence Electron number for Lithium? • 1 • Oxygen? • 6 Here’s a short-cut! Lewis Dot Structures • Only use the element’s symbol and the valence electrons. • Write the symbol and surround it with the correct number of valence electrons. Lithium Lewis Dot Bohr Model Oxygen Lewis Dot Bohr Model