Bohr Model & Lewis Dot Diagrams
advertisement

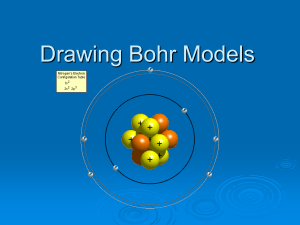
Bohr Model & Lewis Dot Diagrams Both are valuable in a different way! Recall... There are two models of the atoms we will be using in class. Bohr Model Lewis Dot Structure Bohr Model The Bohr Model shows all of the particles in the atom. In the center is circles. Each circle represents a single neutron or proton. Protons should have a plus or P written on them. Neutrons should be blank or have an N. In a circle around the nucleus are the electrons. Electrons should have a minus sign or an e. You can’t just shove all of the electrons into the first orbit of an electron. Electrons live in something called shells or energy levels. Only so many can be in any certain shell. The electrons in the outer most shell of any element are called valance electrons. Draw a Bohr Model for Lithium Drawing a Lithium atom 1. look at the Periodic Table determine the number of protons & neutrons (protons = the atomic number) (neutrons = Atomic mass minus number of protons) determine the number of electrons (electrons match the number of protons for neutral atoms) 2. add protons, neutrons and electrons to their proper positions Try drawing a Bohr Model for the following elements: a) b) c) H He O Bohr Diagrams can become crowded! Lewis Dot Diagrams The Lewis Dot Structure is a bit different from the Bohr model. It only shows the element symbol and it’s outer most electron shell. Step 1: Write the Element Symbol Step 2: Find the number of electrons in the outermost energy level of the Bohr Model (Valence Electrons) Step 3: Place the electrons (dots) around the symbol in clockwise order so that there are no more than 2 electrons per side. Lewis Diagrams solve the “crowding” problem Summary Bohr models are good because they show the energy level of all electrons in an atom. Lewis models are good because they eliminate the “crowded’ picture for elements with many subatomic particles (protons, neutrons, and electrons) Presentation Quiz Try drawing a Bohr Model for the following elements: a) b) c) H He O Try drawing a Lewis Model for those elements: a) b) c) H He O