Lewis Dot Diagrams
advertisement
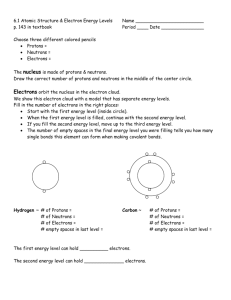
1. It helps to have identified the number of protons, neutrons & electrons an atom has before you try to draw it. You can find this by using this by using isotope notation 2. Isotope notation (shown below for uranium 238) uses information from the Periodic Table to determine the number of protons, neutrons & electrons in an element. 1. 2. 3. The info. on the Periodic Table is rearranged to allow for easy calculation The mass goes at the top left corner of the element’s atomic symbol The number goes at the bottom left corner. 4. The atomic number is equal to the number of protons & electrons, too. 5. The number of neutrons is equal the mass minus the number Using Uranium 238 as our example, the number of both protons and electrons is equal to 92, which is the atomic number. The number of neutrons is equal to 238-92 or 146, which is the atomic mass minus the atomic number. 146 Bohr diagrams show all the protons & neutrons found in the nucleus, and all the electrons in all the energy levels of the electron shell. A simplified Bohr diagram uses the chemical symbol of the element in the center, instead of showing all the protons & neutrons. It still shows all the atoms in all the occupied shells. Li Lewis dot diagrams show only the outermost (valence) electrons. Valence electrons are only found in the outermost shell of an atom. The periodic table helps you to predict how many valence electrons an element will have. Shells fill up in this order: Shell 1st 2nd 3rd etc. # of electrons it holds 2 8 18 So examples of dot diagrams look like this: Lewis dot diagrams can also show how chemical bonds form between atoms: electrons from electrons from hydrogen are yellow oxygen are red If you remember that it takes two electrons to make a chemical bond, then molecules can also be shown as: Double bonds can be shown, too double bond Forming ionic bonds can be represented as: .. to give Na+ :Cl:.. Forming covalent bonds can be shown as