drawing bohr models
advertisement

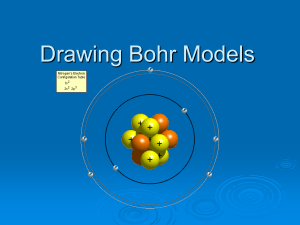
Bohr Model Starter: What do you picture when you think of an atom? (What does it look like?) Practice: Bohr’s Model of the Atom How to draw the model Bohr’s Discovery • Bohr observed the light spectrum of Hydrogen. If all of the electrons existed anywhere in the cloud, he would expect to see a Continuous Spectrum like this: How Bohr Got This • When Bohr observed the light spectrum for Hydrogen, he saw this: What this meant was • The atom was more like this: What it means • This Line Spectrum shows that electrons can only exist at certain levels he called Shells or Orbits. • The electrons can jump from orbit to orbit, but can’t exist in between. • More energy, different color, different line, different energy level. • The more energy an electron has, the higher energy level (orbit) it goes in. Bohr’s Atomic Theory The Bohr model was a modification of the Rutherford model. Bohr however put the electrons in certain circular orbits around the nucleus called shells. + Bohr’s Atomic Theory Protons and neutrons are found in the nucleus. Electrons orbit the nucleus in various shells. Everything else is empty space. 1st shell Nucleus 0 Empty space 2nd shell + 3rd shell Bohr’s Atomic Theory Each of the shells has a maximum number of electrons that it can hold. 1st shell 2 electrons Nucleus Empty space + 2nd shell 8 electrons 3rd shell 18 electrons Bohr’s Atomic Theory Ex.1) Draw a Bohr model of Hydrogen-1. Step-1 Draw a circle to represent the nucleus. Step-2 Determine the number of protons and neutrons and place them in the nucleus. Step-3 Draw a circle around the nucleus to represent the electron shell. Step-4 Place the electron in the shell. 1p 0n H-1 e- Bohr’s Atomic Theory Ex.2) Draw a Bohr model of Helium-2. Step-1 Draw a circle to represent the nucleus. Step-2 Determine the number of protons and neutrons and place them in the nucleus. Step-3 Draw a circle around the nucleus to represent the electron shell. Step-4 Place the electron in the shell. e- Helium-2 2p 2n e- Bohr’s Atomic Theory Ex.3) Draw a Bohr model of Lithium-3. Step-1 Draw a circle to represent the nucleus. Step-2 Determine the number of protons and neutrons and place them in the nucleus. Step-3 Draw circles around the nucleus to represent the electron shells. Step-4 Place the electrons in the shells. e- Litium-3 3p 4n e- e- Bohr’s Atomic Theory Ex.4) Draw a Bohr model of Neon-10. Step-1 Draw a circle to represent the nucleus. Step-2 Determine the number of protons and neutrons and place them in the nucleus. Step-3 Draw circles around the nucleus to represent the electron shells. Step-4 Place the electrons in the shells. e- eee- Neon-10 e- 10p 10n e- e- e- e e- Application Using the White boards you build the following atoms: Sodium Neon Boron Aluminum Bohr Model of Ions • What is an Ion? – An Atom that has lost or gain one or more electrons, giving it a charge. – The positive charge tells how many electrons have been lost. – The negative charge tells how many electrons have been gain. How is charge shown? • The charge is shown in the upper right hand corner of the Symbol. • Examples: – Na+ - sodium ion has a +1 charge because it lost one electron. – O-2 – oxygen ion (known as oxide) has a -2 charge because it gained two electrons. • The electrons are lost or gained in the outer level, either completely empting it, or filling it to eight electrons. Application • Using the White boards you build the following atoms: • Potassium Ion: K+ • Nitrogen Ion (Nitride): N-3 • Bromine Ion (Bromide): Br• Aluminum Ion: Al3+ Electron Terms • Ground State: All electrons are in the lowest energy level. All electrons are in their proper place. • Excited State: One or More electrons are in a higher energy level (orbit) then they should be. Where Bohr Fails • In the 4th Period, electrons start going to a lower energy level. According to Bohr this shouldn’t happen. • In the 4th Period, the first two electrons go in the 4th Level, but the next ten go in the 3rd Level, then the next six go back in the 4th Level. • Bohr couldn’t explain this. Connection How did “building” your atoms help you understand Bohr’s model? Exit What are the limitations of Bohr’s model?