powerpoint file lecture 3
advertisement
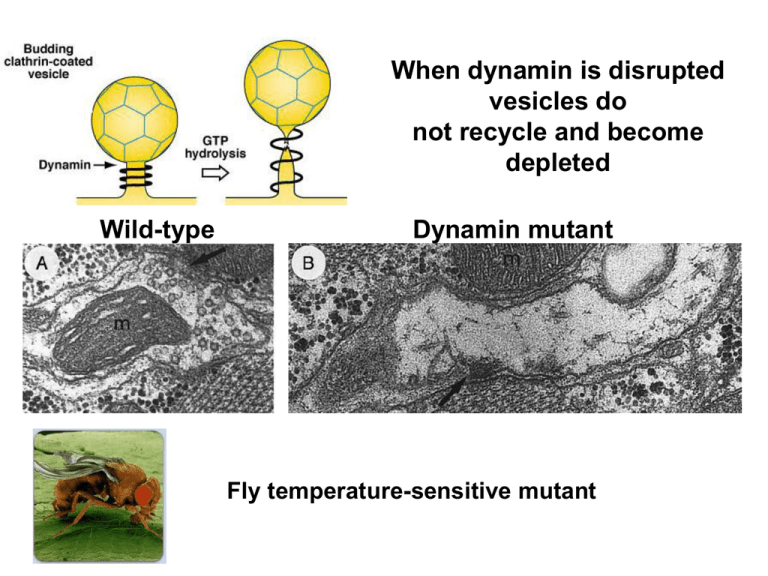
When dynamin is disrupted vesicles do not recycle and become depleted Wild-type Dynamin mutant Fly temperature-sensitive mutant Endocytosis Uncoating Hsc70 Auxilin ATP ADP+Pi Neurological conditions affecting the vesicle cycle Congenital myasthenic syndromes (loss of small vesicles) Lambert-Eaton myasthenic syndrome (antibodies against Ca channels) Clostridial toxins (botulism-paralysis tetanus-muscle spasms) Signaling at the neuromuscular junction (colored electron micrograph) The Neuromuscular Junction The motor neuron axon branches to form multiple synapses (boutons) in the middle of the muscle. Each bouton is physically separated from the muscle endplate by a synaptic cleft 100 nm wide. ACh filled vesicles and Ca2+ channels cluster at active zones A basement membrane lining the cleft contains collagen and acetylcholinesterase an enzyme which hyrolyzes ACh. Each bouton lies above junctional folds in the muscle membrane packed with nicotinic acetylcholine receptors. Depolarization of the muscle membrane initiated by ACh receptor activation, then opens voltage-gated Na+ channels Autoradiograph of neuromuscular junction Black grains indicate binding of radioactive (-bungarotoxin) to ACh receptors enriched at top of junctional folds (10,000 receptors/µm2). -bungarotoxin extracted from the venom of Braided Krait snakes, is a potent inhibitor of some nicotinic ACh receptors Reconstructed image of a nicotinic ACh receptor 8.5nm Pore 0.8nm Receptor is a complex of probably five subunits which together form a channel Toshima and Unwin, 1988 The muscle nicotinic ACh receptor is a typical ionotropic receptor The receptor is made up of five subunits; 2 and 3 non- Binding of 2 ACh molecules opens the channel through a conformational change The inhibitory snake venom -bungarotoxin competes with ACh for binding sites ACh receptor subunits have conserved domains Subunits are 50% conserved suggesting similar structure M2 domains line the channel pore 4 hydrophobic transmembrane domains of 20 amino acids form 4 alpha helixes Functional model of the nicotinic ACh receptor channel Aligned negatively charged amino acids (purple) flanking M2 of each subunit form rings that contribute to Ion selectivity (repulsing anions) Ring of hyrdophobic leucine residues occlude the pore where the M2 is kinked inward Conformational changes are thought to reorientate the residues within the pore, allowing Ions to flow. threonine/serine ring contribute ion selectivity filter Voltage-clamp experiments show the inward synaptic current produced by motor axon stimulation The endplate potential lags behind the ACh generated current due to the charging of the membrane capacitance The ACh receptor is permeable to Na+ and K + At reversal potential the driving forces for Na+ and K+ are equal and opposite so the net ion flow is zero At resting potential the inward driving force for Na+ is large and the outward driving force for K+ is small Na+ K+ Na+ K+ IEPSP=gEPSP X (Vm - EEPSP) Na+ K+ Na+ K+ ACh-gated channels conduct a square-shaped unitary current On-cell patch clamp Erwin Neher Burt Sakmann Unitary current at -90mV is 2.7 pA(10-12A). This current represents the passage of ~17,000 ions The open time varies for individual channels Unitary current at -130mV is 3.9 pA(10-12A). Single open ACh receptors behave as simple resistors The EPSC time course results from the summed contributions of individual ACh-gated channels ACh is released in response to an action potential ACh is removed from cleft rapidly(>1ms) by hydrolosis (acetylcholine esterase) and diffusion Channels close randomly in absence of ACh with a mean open time of 1ms At a typical endplate, the current is -500,000 pA and the single channel current is -2.7 pA so ~ 200,000 individual ACh receptors open to make the smooth endplate current Summary of single-channel currents, end-plate current and end-plate potential Summary of events underlying neurotransmission at the neuromuscular junction