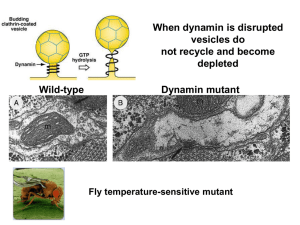
Signal Transduction I
advertisement
I: Signal Transduction Regulation of cell permeability by ligand-gated channels: the nicotinic receptor and other ionotropic receptors Ionotropic vs metabotropic receptors • Opening a channel directly, as is seen in the nicotinic ACh receptor, provides for very rapid transduction of chemical detection into an electrical signal – this is an example of an ionotropic receptor. • As we will see, the muscarinic ACh receptor works in a completely different way…the different subtypes of muscarinic receptors are all metabotropic receptors – in which messenger-receptor binding works by initiating a 2nd message within the target cell. What makes a given synapse excitatory or inhibitory for the postsynaptic cell? It depends on what happens after transmitter binds receptor – not on some intrinsic property of the transmitter chemical itself . Inhibitory Postsynaptic Potentials (IPSPs) All inhibitory mechanisms oppose depolarization Simplest mechanism: increased K+ permeability – which would lead to hyperpolarization Less simple: increased Cl- permeability – sometimes called silent inhibition – tends to stabilize membrane potential at the rest value. Skeletal Muscle Synapse: Nicotinic ACh receptors are the classic example of ligand-gated channels Points from preceding slide: • Accumulation of ACh into the vesicles is driven by a H+ pump. • ACh synthesis occurs in the cytoplasm of the terminal. • The channel that is opened by ACh is called “ligand gated”. Ligand-gated channels are opened or closed by lock-and-key binding with a chemical. • The synaptic potential (end plate potential) is above threshold for an action potential. • The action potential in muscle cells is similar to that in nerve cell axons: Na+ and K+ voltage-gated channels. • The quick recovery from the ACh binding is the result of acetylcholinesterase, which terminates the neurotransmitter effect. Neuromuscular synaptic transmission: effect of ACh on nicotinic receptors of skeletal muscle • 1. A single action potential releases so many vesicles that the depolarization of the muscle cell membrane reaches the threshold for an action potential – transmission is 1:1 – this is a unique synapse! • The nicotinic acetylcholine receptor protein is a ligand-gated cation channel: it is a channel that allows passage of Na+ and K+ when ACh binds. • Because the muscle resting potential is near the K+ equilibrium potential, Na+ is the dominant ion that flows through the channel, exerting a depolarizing effect on the muscle cell membrane. • Patch-clamping reveals that each channel remains open for 2-3 msec., allowing 15,000 – 30,000 Na+ to flow through. The nicotonic ACh receptor is a pentameric channel Toxins that target the ACh receptor have been tools for research • The receptor gets its name from the fact that nicotine mimics the effect of ACh. • a-bungarotoxin is produced by the snake called the banded Krait. Scientists in Taiwan showed that paralysis was the result of binding to the ACh receptor. • Curare is a mixture of plant toxins (purified: tubocarine) used by S.A. Indians for arrowhead poison and in surgery to block muscle reflexes. It blocks the receptor and prevents ACh binding. More examples of ionotropic receptors • The fast synaptic response seen upon activation of nicotinic ACh receptors is similar to the synaptic depolarization initiated by the 2 glutamate ionotropic receptors: both Na+ and K+ can move through the channels. • GABA (gamma amino butyric acid) and glycine receptors are Cl- channels. Their inhibitory effect is to either hyperpolarize the postsynaptic cell by increasing the Cl- permeability (if it is not at equilibrium) and/or to “clamp” the membrane potential at the resting state by making Clmore dominant in determining the resting potential. The GABAA receptor and its binding sites for drugs and modulators Benzodiazepines are antidepressants; barbiturates are depressants; steroids exert an antidepressant effect; pentobarbitol is a ‘local anesthetic’. GABAA receptor: another ligand-gated channel • The GABAA receptor has a hyperpolarizing effect on its target cell which is called an inhibitory postsynaptic potential = IPSP • The effect of benzodiazapene is to increase the Cl- movement through the open channels; this inhibition has a calming effect, as its action is particularly important in brain regions associated with emotional behavior. • Huntington’s chorea is a degenerative disease in which GABA-ergic (GABA-releasing) neurons are lost and the result is uncontrolled movements. The GABA-releasing neurons are thought to die off due to an inherited excessive activity by glutamate-releasing cells, one example of excitotoxicity. Metabotropic receptors • What is a 2nd messenger? Any substance that is released inside the cell or synthesized there in response to messenger-receptor binding at the cell surface, and that effects the target cell’s response to the 1st message. Why 2nd messages? • 2nd messages can do more than just affect the electrical responsiveness of the target cells • 2nd messages reach effectors within the cell and can also affect gene expression • 2nd messages amplify the 1st messagegenerally this is the outcome of a multistage signal cascade. Second messenger amplification increases the ligand’s effect, but this takes time… Opening ion channels is only one of the effects that such second messengers can have. A few of the other pathways are included here. Although not all are present in any one example, more than one change is often activated by binding of the ligand. The 3 major 2nd messenger systems • Cyclic nucleotides (cAMP, cGMP) • Inositol trisphosphate (IP3) • Ca++ – Interaction between these is typical – for example both cyclic nucleotides and IP3 can trigger release of Ca++ G-protein Coupled Receptors • G-Protein coupled receptors, also known as 7-transmembrane or serpentine receptors because 7 a-helices pass through the membrane, are the largest family of transducer proteins, and the largest family of proteins known. The human genome codes for at least 90 Gproteins. • Ligands for the GPCR range from photons through small transmitters to protein signaling molecules of the immune system. • GPCR’s are targets for 40-50% of medicinal drugs A generalized picture of the G-Protein-linked or G-Protein-coupled receptor G Proteins: αβγ = alpha, beta, gamma subunits • G Proteins are molecular switches whose on or off state depends on whether GDT or GTP is bound to the α subunit. (A smaller monomeric G protein is called Ras and is associated with tyrosine kinase receptors that mediate cell growth and movement.) • The G protein moves away from the receptor when GTP binds, and α dissociates from βγ (which are permanently linked). Both pieces of the G protein can interact with messenger systems, although in many cases the βγ subunit’s roles are not known. • When Gα locates its target, the process of activating the enzyme causes hydrolysis of GTP, leaving GDP, and then the αβγ subunits must reunite. This terminates the active response to the ligand. G-Protein Activation One possible target of G protein signal cascades is adenyl cyclase, the enzyme that catalyzes the formation of cyclic AMP Formation of cyclic AMP: the cyclic AMP is destroyed by phosphodiesterase, yielding AMP Different G-protein families are coupled to different 2nd messenger pathways • Gi inhibits the operation of adenyl cyclase • Gs stimulates the operation of the same enzyme • Gq stimulates phospholipase C, resulting in formation of inositol trisphosphate (IP3) and diacylglycerol (DAG) from a common membrane phospholipid, inositol bisphosphate. Summary: Comparison of Ionotropic and Metabotropic receptor activity: metabotropic pathways can either open or close channels Now to the muscarinic receptors • Muscarinic receptors are characteristically found on targets for the parasympathetic division of the autonomic nervous system • 5 subtypes M1-M5 have been identified genetically, but at present pharmacologists can distinguish only 4 subtypes. • The subtypes differ in their locations in the body, their effects (excitatory vs inhibitory) and the particular G-proteins and 2nd messenger systems they are coupled to. All muscarinic receptors are coupled to one of two main 2nd messenger systems M1 Salivary glands, stomach, CNS Gq coupled to phospholipase C: inositol trisphosphate and diacylglycerol; increases intracellular Ca++ M2 Heart, CNS Gi coupled to inhibition of adenylyl cyclase, decreased cAMP M3 Blood vessels, eye, visceral smooth muscle, lung airway Gq coupled to phospholipase C: inositol trisphosphate and diacylglycerol M4 CNS Gi coupled to inhibition of adenylyl cyclase, decreased cAMP M5 ? Gq coupled to phospholipase C: inositol trisphosphate and diacylglycerol Muscarinic receptors are targets for many drugs and toxins – only a few are shown here • M1: carbachol, atropine, scopolamine, mambatoxin MT7 • M2: methachol, carbachol, atropine • M3:, carbachol, methachol, atropine • M4: bethanechol, carbachol, atropine, mambatoxin MT3 Agonists are shown in black; antagonists in red Adrenergic receptors • Adrenergic receptors are found on the targets of the sympathetic division of the autonomic nervous system. • The transmitter released at sympathetic synapses is norepinephrine (noradrenaline); the sympathetic hormone released by the adrenal medulla is epinephrine (adrenaline) – these are catecholamines. • There are 2 families of adrenergic receptors, alpha and beta. • There are 2 subgroups of alpha receptors and 3 of beta receptors Gi coupled Gq coupled Gs coupled