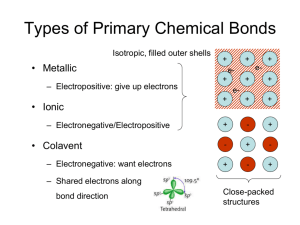
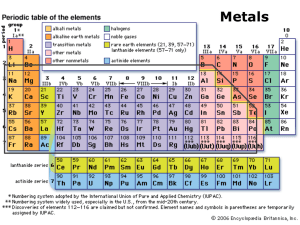
MS115a Lecture 05 10-06
advertisement

Ionic Bonding & Structures • Isotropic bonding • Maximize # of bonds, subject to constraints – Maintain stoichiometry; Alternate anions and cations – Like atoms should not touch ‘Radius Ratio Rules’ – – – – + – – – – – – – + – – – + – – – – Just barely stable Cubic Coordination: CN = 8 2 RA a 2( RA rc ) 3a 2(rc + RA) a rc RA RA 3 rc 3 1 0.732 RA 2RA Cuboctahedral: CN = 12 2RA rc + RA rc + RA = 2RA rc = RA rc/RA = 1 Radius Ratio Rules CN (cation) 2 Geometry linear min rc/RA (f) 3 trigonal planar 0.155 4 tetrahedral 0.225 6 octahedral 0.414 8 cubic 0.732 12 cubo-octahedral 1 none if rc is smaller than fRA, then the space is too big and the structure is unstable Ionic Compounds anion cation Radius Ratio Rules CN (cation) 2 Geometry linear min rc/RA (f) 3 trigonal planar 0.155 4 tetrahedral sites occur within 0.225 close-packed arrays 6 octahedral 0.414 8 cubic 12 cubo-octahedral none common in ionic compounds 0.732 1 if rc is smaller than fRA, then the space is too big and the structure is unstable Local Coordination Structures • Build up ionic structures from closepacked metallic structures • Given range of ionic radii: CN = 4, 6, 8 tetrahedral octahedral occur in closepacked structures HCP: tetrahdral sites 4 sites/unit cell 2 sites/close-packed atom HCP: octahedral sites 2 sites/unit cell 1 site/close-packed atom Sites in cubic close-packed 8 tetrahedral sites/unit cell 2 tetrahedral sites/close-packed atom 4 octahedral sites/unit cell 1 octahedral site/close-packed atom Summary: Sites in HCP & CCP 2 tetrahedral sites / close-packed atom 1 octahedral site / close-packed atom sites are located between layers: number of sites/atom same for ABAB & ABCABC Example: ZnS • S2- ~ 1.84 Å; Zn2+ ~ 0.60 – 0.57 Å; – rc/RA = 0.326 – 0.408 • • • • • CN Zn2+ is big enough for CN = 4 4 6 S2- in close-packed array 8 2+ Zn in tetrahedral sites Zn:S = 1:1 ½ tetrahedral sites filled Which close-packed arrangement? – Either! “Polytypism” – CCP: Zinc blende or Sphaelerite structure – HCP: Wurtzite structure f 0.225 0.414 0.732 ZnS: Zinc Blende CCP anions as CP atoms fill 4/8 tetr sites S2y z=½ z=0 x x x x x y z=½ z=1 x ZnS: Zinc Blende S2- Zn2+ CN(S2-) also = 4 RA/rc > 1 S2- certainly large enough for 4-fold coordination Example: CaF2 (Fluorite) • F- ~ 1.3 Å; Ca2+ ~ 1.0 Å; – rc/RA = 0.77 • Ca2+ is big enough for CN = 8 CN f 4 0.225 6 0.414 8 0.732 – But there are no 8-fold sites in close-packed arrays • Consider structure as CCP cations – F- in tetrahedral sites – RA / rc> 1 fluorine could have higher CN than 4 • Ca:F = 1:2 all tetrahedral sites filled • Places Ca2+ in site of CN = 8 • Why CCP not HCP? - same reason as NaCl Fluorite Ca2+ FCN(F-) = 4 CN(Ca2+) = 8 [target]