MS115a Lect 05 10 05 2011
advertisement

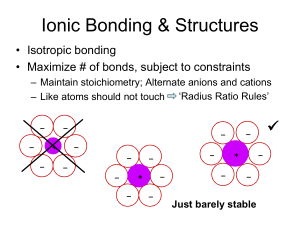
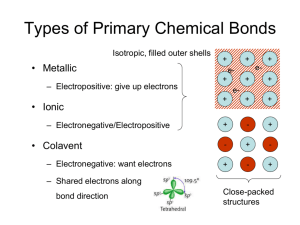
Metals Ionic Compounds anion cation Radius Ratio Rules CN (cation) 2 Geometry linear min rc/RA (f) 3 trigonal planar 0.155 4 tetrahedral sites occur within 0.225 close-packed arrays 6 octahedral 0.414 8 cubic 12 cubo-octahedral none common in ionic compounds 0.732 1 if rc is smaller than fRA, then the space is too big and the structure is unstable Summary: Sites in HCP & CCP 2 tetrahedral sites / close-packed atom 1 octahedral site / close-packed atom sites are located between layers: number of sites/atom same for ABAB & ABCABC Common Ionic Structure Types • Rock salt (NaCl) sometimes also ‘Halite’ – Derive from cubic-close packed array of Cl- • Zinc blende (ZnS) – Derive from cubic-close packed array of S= • Fluorite (CaF2) – Derive from cubic-close packed array of Ca2+ • Cesium chloride (CsCl) – Not derived from a close-packed array • Complex oxides – Multiple cations Example: CaF2 (Fluorite) • F- ~ 1.3 Å; Ca2+ ~ 1.0 Å; – rc/RA = 0.77 • Ca2+ is big enough for CN = 8 CN f 4 0.225 6 0.414 8 0.732 – But there are no 8-fold sites in close-packed arrays • Consider structure as CCP cations – F- in tetrahedral sites – RA / rc> 1 fluorine could have higher CN than 4 • Ca:F = 1:2 all tetrahedral sites filled • Places Ca2+ in site of CN = 8 • Why CCP not HCP? - same reason as NaCl Fluorite Ca2+ FCN(F-) = 4 CN(Ca2+) = 8 [target] CsCl • Cl- ~ 1.8 Å; Cs+ ~ 1.7 Å; – rc/RA = 0.94 • Cs+ is big enough for CN = 8 – But there are no 8-fold sites in close-packed arrays • CsCl unrelated to close-packed structures – Simple cubic array of anions – Cs+ in cuboctahedral sites – RA / rc> 1 chlorine ideally also has large CN • Ca:Cl = 1:1 all sites filled Cesium Chloride Cl- 1 Cs+/unit cell 1 Cl-/unit cell CN(Cs) = 8 Cs+ Why do ionic solids stay bonded? electrostaic • Pair: attraction only E pair Z1Z 2 e 2 4 o r • Solid: repulsion between like charges • Net effect? Compute sum for overall all possible pairs Madelung Energy electrostatic E solid cluster 1 2 i Can show electrostatic E solid N0 j ZiZ je 4 o rij ( Z e ) 4 o r 2 2 Sum over a cluster beyond which energy is unchanged For simple structures Single rij |Z1| = |Z2| = Madelung constant Structures of Complex Oxides • Multiple cations – Perovskite • Capacitors • Related to high Tc superconductors – Spinel • Magnetic properties • Covalency – Zinc blende • Semiconductors – Diamond • Semiconductors – Silicates • Minerals Perovskite – Perovskite: ABO3 [B boron] • A2+B4+O3 A3+B3+O3 A1+B5+O3 • CaTiO3 LaAlO3 KNbO3 above/below • Occurs when RA ~ RO and RA > RB • Coordination numbers A – CN(B) = 6; CN(A) = 12 – CN(O) = 2B + 4A • CN’s make sense? e.g. SrTiO3 – RTi = 0.61 Å – RSr = 1.44 Å – RO = 1.36 Å O B RTi/RO = 0.45 RSr/RO = 1.06 http://abulafia.mt.ic.ac.uk/shannon/ptable.php Tolerance factor close-packed directions A B Covalent Compounds sp3 s2p1 s2p2 s2p3 s2p4 s2 Covalent Structures Recall: zinc blende both species tetrahedral ZnS: +2 -2 or sp3 GaAs: +3 -3 single element: C or Si or Sn diamond Structural Characteristics • Metals – Close-packed structures (CN = 12) – Slightly less close-packed (CN = 8) • Ionic structures – Close-packed with constraints – CN = 4 to 8, sometimes 12 • Covalent structures – Not close-packed, bonding is directional • Any can be strongly or weakly bonded (Tm) Diamond vs. CCP 8 atoms/cell, CN = 4 4 atoms/cell, CN = 12 ½ tetrahedral sites filled 2a 4R 3a 8 R 3 3 8 3 3 V a R 98.5 R 3 3 V / ato m 1 2 .3 R 3 4 3 3 3 V a R 22.6 R 2 V / ato m 5 .7 R 3 Computing density • Establish unit cell contents • Compute unit cell mass • Compute unit cell volume – Unit cell constant, a, given (or a and c, etc.) – Or estimate based on atomic/ionic radii • Compute mass/volume, g/cc • Example: NaCl – – – – Contents = 4 Na + 4 Cl Mass = 4(atom mass Na + atomic mass Cl)/No Vol = a3 Avogardo’s # g / m ol 3 Units = cm 3 # / m o l g / cm Cl Na Single Crystal vs. Polycrystalline Rb3H(SO4)2 Diamond Quartz (SiO2) Ba(Zr,Y)O3-d Periodicity extends uninterrupted throughout entirety of the sample External habit often reflects internal symmetry Regions of uninterrupted periodicity amalgamated into a larger compact = grains delineated by grain boundaries Isotropic vs. Anisotropic graphite* polycrystalline averaging * http://www.electronics-cooling.com/assets/images/2001_August_techbrief_f1.jpg diamond