MS115a Lect 04&5a 10 08 2012
advertisement

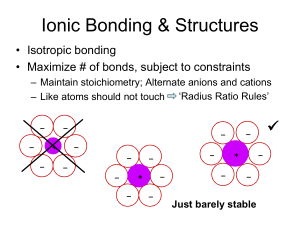
Types of Primary Chemical Bonds Isotropic, filled outer shells • Metallic – Electropositive: give up electrons • Ionic – Electronegative/Electropositive • Colavent – Electronegative: want electrons + + e- e+ + + + + + + + - + - + - + - + e- – Shared electrons along bond direction Close-packed structures Review: Common Metal Structures hcp ABABAB ccp (fcc) ABCABC bcc not close-packed Features • Filled outer shells spherical atom cores, isotropic bonding • Maximize number of bonds high coordination number • High density Metals • single element, fairly electropositive • elements similar in electronegativity Ionic Compounds • elements differing in electronegativity cation anion Ionic Bonding & Structures • Isotropic bonding • Maximize packing density • Maximize # of bonds, subject to constraints – Like atoms should not touch – Maintain stoichiometry – Alternate anions and cations Ionic Bonding & Structures Isotropic bonding; alternate anions and cations – – – + – – – – – – – – – – – + – – + – – Just barely stable Radius Ratio “Rules” Cubic Coordination: CN = 8 2 RA a 2( RA rc ) 3a 2(rc + RA) a rc RA RA 3 rc 3 1 0.732 RA 2RA Cuboctahedral: CN = 12 2RA rc + RA rc + RA = 2RA rc = RA rc/RA = 1 Radius Ratio Rules CN (cation) 2 Geometry min rc/RA none (linear) 3 0.155 (trigonal planar) 4 0.225 (tetrahedral) CN 6 Geometry min rc/RA 0.414 (octahedral) 8 0.732 (cubic) 12 1 (cuboctahedral) Ionic Bonding & Structures • Isotropic bonding • Maximize # of bonds, subject to constraints – Like atoms should not touch • • • • ‘Radius Ratio Rules’ – rather, guidelines Develop assuming rc < RA But inverse considerations also apply n-fold coordinated atom must be at least some size – Maintain stoichiometry • Simple AaBb compound: CN(A) = (b/a)*CN(B) – Alternate anions and cations Radius Ratio Rules CN (cation) 2 Geometry linear min rc/RA (f) 3 trigonal planar 0.155 4 tetrahedral sites occur within 0.225 close-packed arrays 6 octahedral 0.414 8 cubic 12 cubo-octahedral none common in ionic compounds 0.732 1 if rc is smaller than fRA, then the space is too big and the structure is unstable Local Coordination Structures • Build up ionic structures from closepacked metallic structures • Given range of ionic radii: CN = 4, 6, 8 tetrahedral octahedral occur in closepacked structures HCP: tetrahedral sites 4 sites/unit cell 2 sites/close-packed atom HCP: octahedral sites 2 sites/unit cell 1 site/close-packed atom Sites in cubic close-packed 8 tetrahedral sites/unit cell 2 tetrahedral sites/close-packed atom 4 octahedral sites/unit cell 1 octahedral site/close-packed atom Summary: Sites in HCP & CCP 2 tetrahedral sites / close-packed atom 1 octahedral site / close-packed atom sites are located between layers: number of sites/atom same for ABAB & ABCABC Common Ionic Structure Types • Rock salt (NaCl) sometimes also ‘Halite’ – Derive from cubic-close packed array of Cl- • Zinc blende (ZnS) – Derive from cubic-close packed array of S= • Fluorite (CaF2) – Derive from cubic-close packed array of Ca2+ • Cesium chloride (CsCl) – Not derived from a close-packed array • Complex oxides – Multiple cations Example: NaCl (rock salt) • Cl- ~ 1.81 Å; Na+ ~ 0.98 Å; rc/RA = 0.54 • Na+ is big enough for CN = 6 – also big enough for CN = 4, but adopts highest CN possible • Cl- in cubic close-packed array • Na+ in octahedral sites • Na:Cl = 1:1 all sites filled CN f 4 0.225 6 0.414 8 0.732 Rock Salt Structure Cl Na ccp array with sites shown CN(Cl-) also = 6 RA/rc > 1 Cl- certainly large enough for 6-fold coordination Lattice Constant Evaluation rock salt ccp metal a a R R 4R = 2 a a = 2(RA + rc) > ( 4/2)RA Example: ZnS • S2- ~ 1.84 Å; Zn2+ ~ 0.60 – 0.57 Å; – rc/RA = 0.326 – 0.408 • • • • • CN Zn2+ is big enough for CN = 4 4 6 S2- in close-packed array 8 2+ Zn in tetrahedral sites Zn:S = 1:1 ½ tetrahedral sites filled Which close-packed arrangement? – Either! “Polytypism” – CCP: Zinc blende or Sphaelerite structure – HCP: Wurtzite structure f 0.225 0.414 0.732 ZnS: Zinc Blende CCP anions as CP atoms fill 4/8 tetr sites S2z=0 y z=½ z=1 x x x x x y z=½ x ZnS: Zinc Blende S2- Zn2+ CN(S2-) also = 4 RA/rc > 1 S2- certainly large enough for 4-fold coordination Example: CaF2 (Fluorite) • F- ~ 1.3 Å; Ca2+ ~ 1.0 Å; – rc/RA = 0.77 • Ca2+ is big enough for CN = 8 CN f 4 0.225 6 0.414 8 0.732 – But there are no 8-fold sites in close-packed arrays • Consider structure as CCP cations – F- in tetrahedral sites – RA / rc> 1 fluorine could have higher CN than 4 • Ca:F = 1:2 all tetrahedral sites filled • Places Ca2+ in site of CN = 8 • Why CCP not HCP? - same reason as NaCl Fluorite Ca2+ FCN(F-) = 4 CN(Ca2+) = 8 [target] CsCl • Cl- ~ 1.8 Å; Cs+ ~ 1.7 Å; – rc/RA = 0.94 • Cs+ is big enough for CN = 8 – But there are no 8-fold sites in close-packed arrays • CsCl unrelated to close-packed structures – Simple cubic array of anions – Cs+ in cuboctahedral sites – RA / rc> 1 chlorine ideally also has large CN • Ca:Cl = 1:1 all sites filled Cesium Chloride Cl- 1 Cs+/unit cell 1 Cl-/unit cell CN(Cs) = 8 Cs+ Why do ionic solids stay bonded? • Pair: attraction only E electrostaic pair Z1Z 2e 2 4 o r • Solid: repulsion between like charges • Net effect? Compute sum for overall all possible pairs Madelung Energy electrostatic Esolid cluster Zi Z j e2 1 2 i j 4 o rij Sum over a cluster beyond which energy is unchanged ( Ze) N0 4 o r For simple structures Single rij |Z1| = |Z2| = Madelung constant Can show electrostatic Esolid 2 Structures of Complex Oxides • Multiple cations – Perovskite • Capacitors • Related to high Tc superconductors – Spinel • Magnetic properties • Covalency – Zinc blende • Semiconductors – Diamond • Semiconductors – Silicates • Minerals Perovskite – Perovskite: ABO3 [B boron] • A2+B4+O3 A3+B3+O3 A1+B5+O3 • CaTiO3 LaAlO3 KNbO3 above/below • Occurs when RA ~ RO and RA > RB • Coordination numbers A – CN(B) = 6; CN(A) = 12 – CN(O) = 2B + 4A • CN’s make sense? e.g. SrTiO3 – RTi = 0.61 Å – RSr = 1.44 Å – RO = 1.36 Å O B RTi/RO = 0.45 RSr/RO = 1.06 http://abulafia.mt.ic.ac.uk/shannon/ptable.php Tolerance factor close-packed directions A B