Chapter 8 - Ionic and Covalent Solids - Structures
advertisement

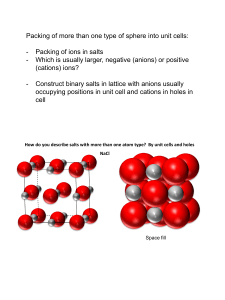
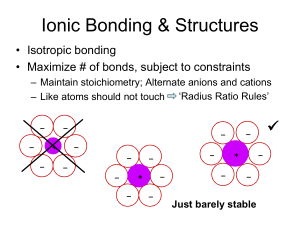
Structures of Ionic and Covalent Solids (Ch. 8) Big-picture perspective: Solids, and in particular inorganic solids, are everywhere around us. Inorganic solids have unique aspects of structure and bonding that contribute to their unique properties and applications, but in many cases these concepts build on what we already know about molecules. We will begin by learning about how the structures of solids are described, and then move into fundamental aspects of chemical bonding in solids. Learning goals: • Describe many crystal structures in terms of close-packed frameworks with systematic filling of octahedral and tetrahedral holes. • Rationalize, using chemical principles, why certain crystal structures are stable for certain compounds but not for others, as well as why certain structural and bonding motifs are preferred for certain compounds relative to others. • Predict which crystal structures are most favorable for a given composition using radius ratio rules and structure maps (and also appreciate the limitations of these approaches). • Predict the preferred formation of normal or inverse spinels using arguments from transition metal chemistry (e.g. crystal field stabilization energies). Structures of solids Crystalline (short-range order that propagates as long-range periodicity) Amorphous (short-range order but no longrange periodicity) Solid State Structures How would you describe the crystal structure of NaCl? Different representations of NaCl Different representations of NaCl Asymmetric unit Lattice points + Crystal structure = Coordination numbers and geometries Coordination number of Na? Cl? Coordination geometries? Coordination polyhedra Coordination polyhedra simplify the view of the structure and emphasize connectivity, but they de-emphasize bonding. Fractional coordinates Fractional coordinates are the positions of atoms in a normalized unit cell Regardless of lattice parameter (a), there are atoms at: Cl (0,0,0) Na (½, 0, 0) Cl (½, 0, ½) Na (0, ½, ½) 1 Cl (1, 0, 0) = (0, 0, 0) Na (½, 1, 0) = (½, 0, 0) 1 0 1 2D projections of 3D structures Projections (slices of the crystal) make it easier to visualize complex structures Niobium oxide Crystal structure related to NaCl but without corner and center atoms • What is the empirical formula of this niobium oxide compound? • How many formula units are contained within the unit cell (molecular formula)? • What are the oxidation states of Nb and O in this compound? • What is the coordination number of Nb? O? • Draw 2D unit cell projections and list fractional coordinates for all atoms “White-Shaded” (W-S) compound • Is this structure related to one of the structures we already studied (primitive cubic, bcc, hcp, fcc)? How? • What is the empirical formula of this W-S compound? • How many formula units are contained within the unit cell (molecular formula)? • What are the oxidation states of W and S in this compound? • What is the coordination number of W? S? • Draw 2D unit cell projections and list fractional coordinates for all atoms Systematic filling of holes Many inorganic crystal structures are based on close-packed arrays of spheres, with different structures derived by systematically filling the holes between packing atoms with other atoms (“interstitial atoms”). Octahedral holes Octahedral holes Tetrahedral holes Tetrahedral holes Octahedral and tetrahedral holes Tetrahedral holes Octahedral holes Revisiting NaCl How would you “assemble” the NaCl structure by starting with a close-packed lattice of Cl– anions and filling in appropriate holes between the close-packed Cl– anions with Na+ cations? Stacking sequence in NaCl We can write a description of the structure in terms of the stacking sequence of packing and interstitial atoms (look at vertical registry) NaCl structure type Many ionic solids crystallize in the NaCl (rocksalt) structure type All alkali halides (except CsCl, CsBr, CsI – why?) Transition metal monoxides (TiO, VO, … , NiO) Alkali earth oxides and sulfides (MgO, CaO, BaS, …) Carbides and nitrides (TiC, TiN, ZrC, NbC) NaCl structure type Carbides and nitrides (TiC, TiN, ZrC, NbC) What would you predict the properties of these interstitial carbides to be? NaCl-related structures FeS2 (iron pyrite) CaC2 (calcium carbide) NaCl-related structures CaCO3 NbO “Hexagonal” NaCl structure What if we try to build the NaCl structure, except start with an hcp array of close packed atoms (instead of fcc)? NiAs structure type NiAs vs. NaCl structure We already looked at what types of solids crystallize in the NaCl structure type. What would you predict about the types of solids that would prefer the NiAs structure type? Same? Different? Why? Tetrahedral structures So far we have filled only the octahedral holes, but there are also tetrahedral holes. What is the stoichiometry if we fill all of the tetrahedral holes? Octahedral and tetrahedral holes Tetrahedral holes Octahedral holes Tetrahedral structures What does the vertical registry look like? Fluorite (top left) and antifluorite (bottom left) We will focus on the fluorite structure (CaF2), where the packing atom is Ca2+ with interstitial F– http://wikis.lib.ncsu.edu/index.php/Fluorite/Antifluorite (some images on fluorite/antifluorite, here and later pages, via Creative Commons license) Fluorite structure type Fluorite-like structures PtN2 PbO K2PtCl6 HgI2 Hexagonal fluorite? NiAs is the hexagonal analogue of NaCl. Is there a hexagonal analogue of fluorite? Zincblende structure type http://www.che.kyutech.ac.jp/chem24/hp/english/lecture/crystal%20structure/zincblende/zincblende.htm Zincblende vs. diamond How does zincblende compare to diamond? ZnS (zincblende) vs. Fluorite How is ZnS (zincblende) related to fluorite? CaF2 (fluorite) Zincblende structure type How would you derive zincblende from filling holes in a close packed lattice? Zincblende vs. wurtzite Compare and contrast Zincblende vs. wurtzite Compare and contrast (boat) (chair) Zincblende vs. wurtzite What would you predict about the types of compounds that form zincblende vs. wurtzite? Zincblende vs. wurtzite Zincblende and wurtzite are examples of polymorphs (Diamond and graphite are allotropes, which are elemental polymorphs) ccp hcp GaAs What would you predict to be the structure of GaAs? Why? Silicon (diamond structure) How many atoms per cell? GaAs GaSe What would you predict to be the structure of GaSe? Why? GaSe As What would you predict to be the structure of As? Why? Semiconductor structures Layered structures Fractional filling of tetrahedral and octahedral holes usually does not occur randomly, and often occurs in layers. CdCl2 (left) vs. CdI2 (right) What types of properties would you expect from these solids? CdCl2 vs. CdI2 Based on the structures, what would you predict about the types of compounds that would form the CdCl2 and CdI2 structure types? Physical and chemical properties Layered structure tend to make plate-like crystals that are soft and slippery (solid-state lubricants). They cleave easily along van der Waals planes, and undergo interlayer chemical reactions (“intercalation”) Going deeper … TiS2 vs. FeS2 If TiS2 is layered, why is FeS2 a three dimensionally bonded structure (related to NaCl), despite the same 1:2 formula? TiS2 (CdI2 structure type) FeS2 (pyrite structure type) TiS2 vs. FeS2 TiS2 vs. MoS2 Both are layered solids, but differ in how the sulfur atoms are oriented. Why? MoS2 TiS2 TiS2 vs. MoS2 Structure prediction By now we have seen several types of crystal structures, and there are many many more. How do we know when a certain compound will adopt a particular structure? Step back – what factors lead to the formation of a particular structure? Consider the simplest structures we’ve seen: MX: NaCl, CsCl, ZnS (zincblende / wurtzite) MX2: CaF2, rutile CsCl structure type Rutile structure type Ionic structure stabilization Structures are stabilized by maximizing anion / cation contact. We can estimate the “best fit” from ionic radii (e.g. geometry, hard sphere close packing) Radius ratio rules Coordination number Geometry r+/r– Radius ratio rules While this model is simple and has its limitations and shortcomings, it can be used as one of several guidelines for structure prediction. It predicts the following correctly: SiO2, BeF2 CN = 4 TiO2, MgF2 CN = 6 ZrO2, CaF2 CN = 8 Despite this success, though, it gets half of the simple MX halides wrong!!! It predicts that LiCl and LiBr should be ZnS-type and KF should be CsCl type! Analogous to our bonding models, we need a more sophisticated approach… Structure maps A more successful approach is based on periodic trends – electronegativity Structure maps Mooser-Pearson plot correctly differentiates alkali halides, and suggests that radius ratio correlations may be coincidental… Spinel structure Two views of the spinel crystal structure Spinel structure Another view of the spinel crystal structure Spinel structure ccp array of Xn– anions (often O2–) 1/8 of the tetrahedral holes filled (“A” sites) 1/2 of the octahedral holes filled (“B” sites) Formula: AxByOz What are x, y, and z? What is the empirical formula for a spinel? Spinel structure What would you predict to be common A-B combinations, and why? The mineral spinel The mineral spinel is a “normal” spinel… Inverse spinels What is an inverse spinel? How would we know whether a spinel is “normal” or “inverse”? How would we predict whether a certain A-B combination would prefer to form as a “normal” or as an “inverse” spinel? Why would the difference between “normal” and “inverse” matter? Spinels and CFSE Spinels have cations occupying both tetrahedral and octahedral sites. Consider a metal cation in a tetrahedral site… Spinels and CFSE Consider a metal cation in an octahedral site… Fe3O4 (magnetite) Is Fe3O4 a normal or an inverse spinel? To begin: What is the oxidation state of Fe in Fe3O4? What 3dn electron configuration(s)? Fe3O4 Octahedral vs. tetrahedral sites Look for the d-orbital occupancy configurations that give the highest CFSE NiFe2O4 Is NiFe2O4 a normal or an inverse spinel? Chromite spinels Is MIICr2O4 a normal or an inverse spinel? Experimental validation How do we probe experimentally whether these spinels are normal or inverse (e.g. that one cation prefers the tetrahedral site and another prefers the octahedral site)? Superexchange Coupling between the 3d electrons of Fe3+ cations on tetrahedral and octahedral sites is through oxygen 2p electrons – “superexchange” Magnetic coupling What type of magnetic coupling is present? Other systems exhibiting superexchange NiO TiO