Presentation 1
advertisement

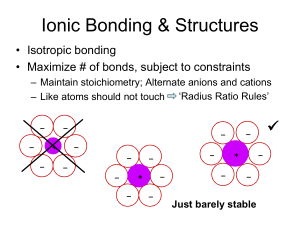
Minerals Ionic Solids Types of bonds Covalent bonding e-s shared equally Ionic coulombic attraction between anion and cation e-s localized Ionic / covalent character depends on difference in electronegativity Electronegativity Calculate stabilities of A+B- and A-B+ Difference in energies given by E(A+B-) – E(A-B+) = (IPA – EAB) – (IPB – EAA) = (IPA + EAA) – (IPB + EAB) According to Mulliken half the above difference is the difference in electronegativities of A and B. Thus, the electronegativity of either is ½ (IP + EA) Ionization Potential IP = energy required for A A+ + e- Electron Affinity EA = energy released in A + e A- What is meant by the statements that Si – O has 50 % ionic character or that Al – O has 60 % ionic character? F Cs = 3.98, most electronegative = 0.79, least O Si Al = 3.44 = 1.90 = 1.61 (3.44 – 1.90) / (3.98 – 0.79) = 0.48 (3.44 – 1.61) / (3.98 – 0.79) = 0.57 Derive minimum radius ratio, r+ / r- for coordination 6 If r- = 1, r+ + r- = 21/2. So, r+ / r- = 0.414. Approach for coordination #s 8 and 12 is similar. That for #4 is trickier. Maybe think, equilateral (tetrahedral) pyramids. Derive minimum radius ratios for coordination 4, 8 and 12 Isomorphic substitution of Na+ by Ca2+ is much more common than Na+ for K+. Similarly, Li+ replaces Mg2+ more often than it replaces Na+. Use ionic radii to explain. See Table 2.1. This is just a matter of size compatibility. Show that OH- in below structure for gibbsite satisfies Pauling Rule 2. s = Z / CN = 3 / 6 = 1 / 2. Σ s = ½ + ½ = ABS(-1), for OH-1 which is consistent with the above structure. Use the equation s = Z / CN (where s is bond strength, Z is cation valance and CN is coordination number) and Pauling Rule 2 to show that a corner of a Si – O tetrahedron can be linked to one other Si – O tetrahedron but not solely to one other Al – O tetrahedron. In the latter case, show that either two monovalent cations or one bivalent cation with CN = 8 are needed to satisfy the rule. In the first case, s = 4 / 4 = 1, and Σ s = 1 + 1 = 2 = ABS(-2), for O2-. However, in the second case Σ s = 1 + ¾ = 1 ¾ so that additional bond(s) are needed to satisfy Rule 2. Conceivably, this might involve either 1) 1/8 + 1/8 = 1/4 or 2) 2/8 = 1/4. SiO4 held together with bivalent cations Si / O = 0.25, which is low So little covalency, easily weathered Si2O6 held together with bivalent cations in octahedral coordination Single chains Si4O11 held together with bivalent cations in octahedral coordination Isomorphic substitution of Al for Si occurs Double chains Si2O5 as Si tetrahedral sheet fused to M octahedral sheet Bonding via apical O of Si tetrahedral sheet M = Al, Fe or Mg, typically coordinated to O2- or OHIsomorphic substitution of Al for Si and Al or Fe for Mg Biotite and muscovite common K+ balances excess negative charge arising from substitution Located in holes of opposing Si tetrahedral sheets What is the coordination number for K+? It fits here, 6Os from the 2 adjacent Si tetrahedral sheets. Therefore, CN = 12. Weathering of muscovite, congruent dissolution K2[Si6Al2]Al4O20(OH)4(s) + 6C2O4H2(aq) + 4H2O = 2K+ + 6C2O4Al+(aq) + 6Si(OH)4(aq) + 8OH-(aq) Involves complexation, hydrolysis and loss of silicic acid Weathering of muscovite, incongruent dissolution K2[Si6Al2]Al4O20(OH)4(s) + 0.8Ca2+(aq) + 1.3Si(OH)4(aq) = 2K+(aq) + 0.4OH-(aq) + 1.6H2O + 1.1Ca0.7[Si6.6Al1.4]Al4O20(OH)4(s) Involves reduction of interlayer charge and cation exchange Weathering of biotite to vermiculite K2[Si6Al2]Mg4Fe(II)2O20(OH)4(s) + 3Mg2+(aq) + 2Si(OH)4(aq) = 1.25Mg0.4[Si6.4Al1.6]Mg5.2Fe(III)0.8O20(OH)4(s) + FeO(OH)(s) + 2K+(aq) + 4H+(aq) Involves Fe oxidation, also reducing interlayer charge What is the interlayer charge of muscovite, cmol(+) / kg? K2[Si6Al2]Al4O20(OH)4 2 moles of – charge per unit formula due to substitution of Al3+ for Si4+. Therefore, 200 cmol(+) / mass of unit formula (kg) AlSi3O8- or Al2Si2O82- in 3-D framework with mono- or divalent cations balancing negative charge Isomorphic substitution, Al for Si NaAlSi3O8(s) + 8H2O(l) = Al(OH)3(s) + Na+(aq) + 3Si(OH)4(aq) + OH-(aq) Weathering of albite to gibbsite involves hydrolysis 4KAlSi3O8(s) + 0.5Mg2+(aq) + 2H+(aq) + 10H2O(l) = K[Si7.5Al0.5]Al3.5Mg0.5O20(OH)4(s) + 4.5Si(OH)4(aq) + 3K+(aq) Weathering of orthoclase to montmorillonite involves acidic hydrolysis Generally, weathering of primary silicates involves Loss of tetrahedrally coordinated Al Oxidation of Fe2+ Consumption of H+ Release of silicic acid and cations Phyllosilicates Dominate clay fraction for intermediate to advanced weathering stage Si tetrahedral and Al / Mg octahedral sheets Bonding via apical Os creates distortion –imperfect fit, corners of octahedra and hexangonal structure in Si tetrahedral sheet 1:1 kaolin and serpentine Dioctahedral, kaolin (common in soil) Trioctahedral, serpentine (rare) Little isomorphic substitution Kaolin Kaolinite crystalline units H-bonded together Halloysite interlayer includes structural water but will dehydrate Morphology usually tubular Ormsby et al. (1962) fractionated kaolinites and found Sample # Particle-size fraction (micrometer) 44-10 10-5 2-1 1-0.5 ________________________________________________________ A ------------------------------ m2 / g --------------------------------5.02 5.86 8.60 8.80 H 6.09 6.59 8.86 10.06 Calculate the surface area for kaolinite of 10 and 1 micrometer equivalent spherical diameter and compare to the above. Assume a density of 2.63 g / cm3 (Deeds and van Olphen, 1963) A / ρV = 3 / ρr which with r = 5 x 10-4 and 5 x 10-5 cm, respectively, gives 3 / 1.325 x 10-3 = 2260 cm2 / g or 0.226 m2 / g and 2.26 m2 /g, respectively, which are less than in Ormsby et al. (1962). See kaolinite figure (A and B). Deviation from minimum surface area (sphere) increases with increasing size fraction. Which serpentine is suspected of causing cancer? 2:1 pyrophyllite and talc Dioctahedral, pyrophyllite Trioctahedral, talc Negligible isomorphic substitution Essentially ideal structures 2:1 smectite and saponite Dioctahedral, smectite Trioctahedral, saponite Smecites differentiated Based on site of isomorpthic substitution Montmorillonite, substitution predominantly in octahedral Beidellite, substitution in tetrahedral Nontronite, substitution in tetrahedral and Fe3+ dominant in octahedral Saponites differentiated based on site of isomorphic substitution Saponite, substitution in tetrahedral sheet Hectorite, substitution in octahedral sheet Also, include presence of Li+ 2:1 vermiculite Include dioctahedral and trioctahedral forms Dioctahedral forms exhibit isomorphic substitution in both tetrahedral and octahedral sheets whereas Trioctahedral forms exhibit substitution in tetrahedral sheet Mg2+ is the octahedral cation Typically form from micas Weathering of biotite to vermiculite K2[Si6Al2]Mg4Fe(II)2O20(OH)4(s) + 3Mg2+(aq) + 2Si(OH)4(aq) = 1.25Mg0.4[Si6.4Al1.6]Mg5.2Fe(III)0.8O20(OH)4(s) + FeO(OH)(s) + 2K+(aq) + 4H+(aq) 2:1 illite (hydrous mica and other names) Dioctahedral mineral similar to and weathered from mica, including K+ as the dominant interlayer cation but Less subsitution in tetrahedral sheet (more Si) Less K+ Oxides, oxyhydroxides and hydroxides Al Al(OH)3 AlOOH Fe FeOOH FeOOH Fe3O4 Fe2O3 Fe2O3 Problems 7, 10, 11, 12 and 14