ppt: RSIEEAE
advertisement

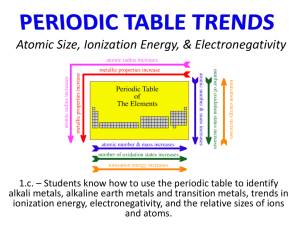
Relative Size Ionization Energy Electron Affinity Electronegativity General Rule - Relative Size When looking at the periodic table, atoms get bigger: Top to bottom Right to left http://www.lung-cancer-report.com/berylliosis-beryllium/picture-of-beryllium-atom.html Top to Bottom Down a Column The atoms get bigger because the electrons are in a higher energy level. Left to Right Across a Row The atoms get smaller because the positive charge in the middle of the atom is more concentrated so it’s sucking the electrons in closer. Relative Size Chart http://www.iun.edu/~cpanhd/C101webnotes/modern-atomic-theory/atomic-radii.html *Positive Ions and Negative Ions* Positive Ions: If an atom loses all of its valence (outermost) electrons, it’s going down to the next energy level so it’s getting smaller. Losing electrons = positive charge More protons than electrons = + charge *Positive Ions and Negative Ions* Negative Ions: If an atom gains electrons it has a negative charge. The nucleus stays the same but the radius gets bigger because there are more electrons. Gaining electrons = negative charge More electrons than protons = charge Sample Problems - Part 1 Which one has a larger radius? (a) Ti or Ni (b) F or F-1 (c) Ba or Hf (d) Ca or Ca+2 Sample Problems - Part 2 Which one has a smaller radius? (a) K or Cs (b) Cs or Os (c) O or O-2 (d) Na or Na+1 Challenge Excluding the Noble Gases because they’re special: What is the smallest atom? ____________________ What is the largest atom? ____________________ Ionization Energy Definition: The energy required to remove an electron from an atom. First Ionization Energy The energy needed to remove the most loosely held electron from an atom. Measured in kilojoules per mole (kj/mol). General Rule Ionization Energy Ionization Energy increases going across periods (rows) - increases as atomic number increases. Ionization Energy decreases going down groups (columns). Classifying Elements based on Ionization Energy Metals Tend to lose electrons to become more stable Low ionization energy •Nonmetals •Tend to gain electrons to become more stable •High ionization energy Noble Gases Have an octet so they’re already stable Very high ionization energy Electron Affinity Definition: the attraction of an atom for an additional electron Metals tend to have low electron affinities. Nonmetals tend to have high electron affinities. Sample Problems - Part 3 Which one has a lower ionization energy? (a) Cl or Kr (b) Iron or Iodine Which one has a higher electron affinity? (a) Oxygen or Barium (b) Na or Cl Electronegativity Definition: the relative attraction of an atom for a shared pair of electrons. The difference in electronegativities increases as the bond strength between two atoms increases. • Metals tend to have low electronegativities. • Nonmetals tend to have high electronegativities. Electronegativity Chart Quic kTime™ and a TIFF ( Unc ompres s ed) dec ompr ess or are needed to s ee this pic ture. www.monroecc.edu/wusers/flanzafame/PerElNegativity.pdf Sample Problems - Part 4 Which atom has a higher electronegativity? (a) Ca or C (b) Titanium or Tellurium Which atom has a lower electronegativity? (a) Br or Be (b) Strontium or Selenium Factors that affect Ionization Energy, Electron Affinity, and Electronegativity 1. Distance from nucleus 2. Stability Connections between Ionization Energy, Electron Affinity, and Electronegativity In general, as Electron Affinity increases, Ionization Energy increases. Electron Affinity and Electronegativity increase going across a period (left to right). Ionization Energy and Electronegativity increase going up a column (bottom to top).