Per. Table Review Key
advertisement

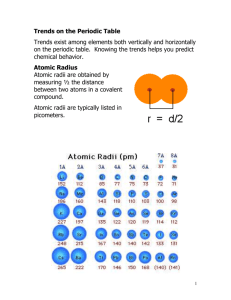
Chapter 13 Periodic Table Review Questions Be able to locate the following: Alkali Metals Explain each of the following: See powerpoint notes Atomic radius Alkaline Earth Elements Shielding effect Halogens Ionization energy Transition elements Electronegativity Noble gases Mendeleev’s method of developing the periodic table Inner transition elements Nuclear charge As you go down a group on the periodic table, what trend do you see in the following – Explain each. Atomic radius Nuclear charge Ionization energies Electronegativity Shielding effect As you go across a period from left to right on the periodic table, what trend do you see in the following – Explain each. Atomic radius Nuclear charge Ionization energies Electronegativity Shielding effect Sample Questions KEY How many valence electrons do elements in Group 1 have? Group 2? Etc… Elements in the representative groups have the same number of valence electrons as the group number. Group 1 has 1, Group 2 has 2, Group3 have 3, etc…. Which element is the most electronegative? P, O, Br, Ca Electronegativities decrease down the groups, and increase as you go from left to right. Therefore Oxygen should be the most electronegative Which element has the largest radius? Na, Cs, N, I Atomic radii increase down the groups and decrease from left to right. The largest radius would be found on Cesium. Which element has the smallest radius? Fr, Cl, Na, Te Choose the element that is nearest the top and to the right side of the table ….Cl Which group of elements experience a small 1st and second ionization energy, but a large 3rd ionization energy? Explain. Since the energies to remove electrons are low for the first 2 electrons, this describes that the elements that have two valence electrons (easy to remove), so we are looking at Group 2 elements. The third electron is very difficult to remove because after it gains the noble gas configuration, the atom is very stable and removing electrons requires much more energy. Which elements tend to become cations? Why? Metals have low electronegativities, give up their electrons freely and become cations Which elements tend to become anions? Why? Non- metals have high electronegativities and attract electrons readily and become anions. Why does Magnesium have an extremely large third ionization energy? As mentioned above, Mg is in group 2, which will give up its 2 valence electrons in order to gain a stable configuration like the noble gasses. Once this occurs, it requires a great deal of energy to remove another electron. Why is the atomic radius of Fluorine smaller than that of Boron? Even though they are in the same period, Flourine has more protons(higher nuclear charge) and pulls the electrons closer to the nuclear Why does Tin have a larger atomic radius than Silicon? Tin is in period 5, which means there are 5 levels of electrons. Si is in period 3, so it has 3 levels of electrons. The higher the period the more energy levels in which the electrons are located, the larger the radius. Why is does Francium have the lowest ionization energy of all the representative elements? Francium is lowest and left-most on the periodic table. It is the most active metal and gives up its electron the easiest. Why does Fluorine have the largest electronegativity of all the representative elements? Flourine is located highest and right-most on the chart (excluding the noble gasses), is the most reactive non-metal and therefore has a high electrongativity. It has a strong attraction to free electrons. Why do the noble gasses have electronegativities of 0? These elements are very stable as the have filled s and p orbitals.(complete set of 8) They do not have attractions to free electrons because they are stable.