Document
advertisement

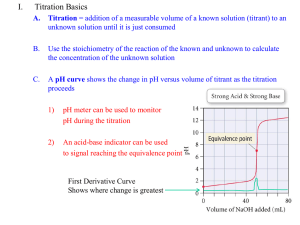
Chapter 10 Acid-Base Titrations A solution containing pure protein, with no other ions present except H+ and OHderived from the protein and water, is said to be isoionic. In medicinal chemistry, the pKa and lipophilicity of a candidate drug predict how easily it will cross cell membranes. 10-1 Titration of Strong Base with Strong Acid Our goal is to construct a graph showing how the pH canges as titrant is added. The titration of 50.00 mL of 0.0200 M KOH with 0.1000 M HBr : H+ + OH- H2O K = 1/KW = 1014 Any amount of H+ added will consume a stoichiometric amount of OH-. (Ve(mL))(0.100 0 M) = (50.00 mL)(0.020 00 M) Ve = 10.00 mL mmol of HBr at equivalence point mmol of OHbeing titrated 1. Before the equivalence point, the pH is determined by excess OH- in the solution. 2. At the equivalence point, H+ is just sufficient to react with all OH- to make H2O. 3. After the equvalence point, pH is determined by excess H+ in the solution. As a reminder, the equivalence point occurs when the added titrant is exactly enough for stoichometric reaction with the analyte. What we actually measure is the end point, which is marked by a sudden physical change, such as indicator color or an electrode potential. Region 1: Before the equivalence Point Region 2: At the Equivalence Point H2O = H+ + OHx x KW = x 2 x = 1.00 X 10-7 M pH = 7.00 As we will soon discover, the pH is not 7.00 at the equivalence point in the tirtration of weak acids or bases. Region 3: After the Equivalence Point Volume of excess H+ Initial concentration of H+ Dilution factor Total volume of solution pH = -log[H+] = 3.08 The Titration Curve The equivalence point is where the slope (dpH/dVa) is greatest ( and the second derivative is 0, which makes it an inflection point). To repeat an important statement, the pH at the equivalence point is 7.00 only in a strongacid-strong-base titration. If one or both of the reactants are weak, the equivalence point pH is not 7.00. 10-2 Titration of Weak Acid with Strong Base The titration reaction is As we saw in Box 9-3, strong plus weak react completely. (Vb(mL))(0.100 0 M) = (50.00 mL)(0.020 00 M) Vb = 10.00 mL mmol of base mmol of HA 1. Before any base is added, the solution contains just HA in water. This is a weak acid whose pH is determined by the equilibrium Ka HA = H+ + A- 2. From the first addition of NaOH until immediately before the equivalence point, there is a mixture of unreacted HA plus the A- produced by Reaction 11-2. Aha! A buffer! We can use the Henderson-Hasselbalch equation to find the pH. 3. At the equivalence point, “all” HA has been converted into A-. The same solution could have been made by dissolving A- in water. We have a weak base whose pH is determined by the reaction A- + H2O Kb = HA + OH- 4. Beyond the equivalence point, excess NaOH is being added to a solution of A-. To a good approximation, pH is determined by the strong base. We calculate the pH as if we had simply added excess NaOH to water. We neglect the tiny effect of A-. Region 1: Before Base Is Added HA = H+ + AF-x x x Ka = 10-6.27 Region 2: Before the Equivalence Point Titration reaction: Relative initial quantities(HA = 1) Relative final quantities Once we know the quotient [A-]/[HA] in any solution, we know its pH: Titration reaction: Relative initial quantities Relative final quantities Advice As soon as you recognize a mixture of HA and A- in any solution, you have a buffer! You can calculate the pH from the quotient [A-]/[HA]. Region 3: At the Equivalence Point A solution of Na+A- is merely a solution of a weak base. A- + H2O = HA + OH- F-x x x Kb = Kw/Ka Initial volume of HA Initial concentration of HA Dilution factor Total volume of solution The pH at the equivalence point in this titration is 9.25. It is not 7.00. The equivalence point pH will always be above 7 for the titration of a weak acid, because the acid is converted into its conjugate base at the equivalence point. Region 4: After the Equivalence Point Volume of excess OH- Initial concentration of OH- Dilution factor Total volume of solution The Titration Curve If you look back at Figure 94b, you will note that the maximum buffer capacity occurs when pH = pKa. It is not practical to titrate an acid or base when its strength is too weak or its concentration too dilute. 10-3 Titration of Weak Base with Strong Acid The titration of a weak base with a strong acid is just the reverse of the titration of a weak acid with a strong base. The titration reaction is B + H+ = BH+ 1. Before acid is added, the solution contains just the weak base, B, in water. The pH is determined by the Kb reaction. Kb B + H2O = BH+ + OHF-x x x 2. Between the initial point and the equivalence point, there is a mixture of B and BH+ㅡAha! A buffer! The pH is computed by using pH = pKa (for BH+) + log([B]/[BH+]) 3. At the equivalence point, B has been converted into BH+, a weak acid. The pH is calculated by considering the acid dissociation reaction of BH+. BH+ = B + H+ F’ – x x x Ka = Kw/Kb The pH at the equivalence point must be below 7. 4. After the equivalence point, the excess strong acid determines the pH. We neglect the contribution of weak acid, BH+. 10-4 Ttitrations in Diprotic Systems A typical Case B + H+ BH+ BH+ + H+ BH22+ (Ve(mL))(0.100 0 M) = (10.00 mL)(0.100 0 M) Ve = 10.00 mL mmol of HCl mmol of B Point A B + H2O 0.100 - x Kb1 = BH+ + OHx x Point B The pH is calculated from the Henderson-Hasselbalch equation for the weak acid, BH+, whose acid dissociation constant is Ka2 (for BH22+) = Kw/Kb1 = 10-10.00 pH = pKa2 + log([B]/[BH+]) = 10.00 + log1 = 10.00 [B]/[BH+] = 8.5/1.5 pH = 10.00 + log(8.5/1.5) = 10.75 Point C At the first equivalence point, B has been converted into BH+, the intermediate form of the diprotic acid, BH22+. BH+ is both an acid and a base. Initial volume of B Original concentration of B Dilution factor Total volume of solution This is the least-buffered point on the whole curve, because the pH changes most rapidly if small amounts of acid or base are added. There is a misconception that the intermediate form of a diprotic acid behaves as a buffer when, in fact, it is the worst choice for a buffer. Point D pH = pKa1 + log([BH+]/[BH22+]) = 5.00 + log1 = 5.00 PointE Original volume of B Total volume of solution BH22+ = BH+ + H+ F-x x x Ka1 = Kw/Kb2 [H+] = (0.100 M)(5.00/35.00) = 1.43 X 10-2 M pH = 1.85 Blurred End Points Titrations of many diprotic acids or bases show two clear end points, as in curve a in Figure 11-4. Some titrations do not show both end points, as illustrated by curve b, which is calculated for the titration of 10.0 mL of 0.100 M nicotine (pKb1 = 6.15, pKb2 = 10.85) with 0.100 M HCl. Nicotine (B) 10-5 Finding the End Point with a pH Electrode Box 10-1 Alkalinity and Acidity Alkalinity is defined as the capacity of natural water to react with H+ to reach pH 4.5, which is the second equivalence point in the titration of carbonate (CO32-) with H+. Alkalinity ≈ [OH-] + 2[CO32-] + [HCO3-] Alkalinity and hardness (dissolved Ca2+ and Mg+, Box 12-3) are important characteristics of irrigation water. Acidity of natural waters refers to the total acid content that can be titrated to pH 8.3 with NaOH. Figure 2-12 shows an autotitrator, which performs the entire operation automatically.4 Figure 11-6a shows two clear breaks, near 90 and 120 µL, which correspond to titration of the third and fourth protons of H6A. H4A2- + OH- H3A3- + H2O (~90µL equivalence point) H3A3- + OH- H2A4- + H2O (~120µL equivalence point) Using Derivatives to Find the End Point Using a Gran Plot to Find the End Point7,8 Gran plot uses data from before the end point (typically from 0.8 Ve or 0.9 Ve up to Ve) to locate the end point. HA = H+ + A- Ka = ([H+]γH+[A-]γA-)/[HA]γHA It will be necessary to include activity coefficients in this discussion because a pH electrode responds to hydrogen ion activity, not concentration. moles of OH- delivered total volume original moles of HA – moles of OHtotal volume Gran plot equation: A graph of Vb10-pH versus Vb is called a Gran plot. The beauty of a Gran plot is that it enables us to use data taken before the end point to find the end point. Challenge Show that when weak base, B, is titrated with a strong acid, the Gran function is (11-6) where Va is the volume of strong acid and Ka is the acid dissociation constant of BH+. 10-6 Finding the End Point with Indicators An acid-base indicator is itself an acid or base whose various protonated species have different colors. K R =1 Y- + H+ pH = pK1 + log([Y-]/[R]) pH 0.7 1.7 2.7 [Y-]:[R] 1:10 1:1 10:1 (11-7) Color red orange yellow The pH range (1.2 to 2.8) over which the color changes is called the transition range. Choosing an Indicator The difference between the observed end point (color change) and the true equivalence point is called the indicator error. Demonstration 10-1 Indicators and the Acidity of CO2 Add 20 mL of 6 M HCl to the bottom of each cylinder, using a length of Tygon tubing attached to a funnel. Box 10-2 What Does a Negative pH Mean? p-Nitroaniline B p-Nitroanilinium ion BH+ (for BH+) (for CH+) (for CH+) (for BH+) The acidity of a solvent that protonates the weak base, B, is defined as the Hammett acidity function: Hammett acidity function: (for BH+) When we refer to negative pH, we usually mean H0 values. Acid H2SO4(100%) H2SO4 · SO3 Name sulfuric acid fuming sulfuric acid (oleum) HSO3F fluorosulfuric acid HSO3F + 10% SbF5 “super acid” HSO3F + 7% SbF5 · 3SO3 ㅡ H0 -11.93 -14.14 -15.07 -18.94 -19.35 In general, we seek an indicator whose transition range overlaps the steepest part of the titration curve as closely as possible. 10-7 Practical Notes Acids and bases in Table 11-5 can be obtained pure enough to be primary standards.17 OH- + CO2 HCO3- 10-8 Kjeldahl Nitrogen Analysis BOX 10-3 Kjeldahl Nitrogen Analysis Behind the Headlines 10-9 The Leveling Effect The strongest acid that can exist in water is H3O+ and the strongest base is OH-. Because of this leveling effect, HClO4 and HCl behave as if they had the same acid strength; both are leveled to H3O+: HClO4 + H2O H3O+ + ClO4- HCl + H2O H3O+ + Cl- HClO4 + CH3CO2H = CH3CO2H2+ + ClO4- K = 1.3 X 10-5 Acetic acid solvent HCl + CH3CO2H = CH3CO2H2+ + ClTitration with HClO4 in H2O: K = 2.8 X 10-9 B + H3O = BH+ + H2O The end point cannot be recognized, because the equilibrium constant for the titration reaction is not large enough. If an acid stronger than H3O+ were available, the titration reaction might have an equilibrium constant large enough to give a distinct end point. Titration with HClO4 in CH3CO2H: B + HClO4 = BH+ClO4An ion pair (The product in this reaction is written as an ion pair because the dielectric constant of acetic acid is too low to allow ions to separate extensively.) 10-10 Calculating Titration Curves with Spreadsheets Titrating a Weak Acid with a strong Base Charge balance: Fraction of titration for weak acid by strong base: [H+] + [Na+] = [A-] + [OH-] We put in a concentration of H+ and get out the volume of titrant that produces that concentration. Cb = 0.1 [H+] = 10-pH Ca = 0.02 [OH-] = Kw/[H+] Va = 50 Ka = 5.37 X 10-7 αA- = Ka/([H+] + Ka) Kw = 10-14 is the input is the output Titrating a Weak Acid with a Weak Base Charge balance: [H+] + [BH+] = [A-] + [OH-] [HA] = αHAFHA αHA = [H+]/([H+] + Ka) [BH+] = αBH · FB αBH = [H+]/([H+] + KBH ) + Fraction of titration for weak acid by weak base: + +