Acid-base titration properties
advertisement

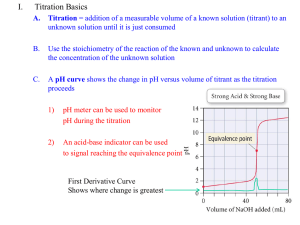
CHEMISTRY 59-320 ANALYTICAL CHEMISTRY Fall - 2010 Lecture 17 Chapter 13: Acid-Base Titrations Introduction • The goal of this chapter is to establish a graph showing how the pH changes as titrant is added. • There are three distinct regions in a titration curve: (1) before the equivalence point, (2) around the equivalence point, and (3) beyond equivalence point. • From an acid-base titration curve, pKa values of acidic and basic substances can be deduced. • Lipophilicity, a measure of solubility in nonpolar solvents, can predict how easily a drug candidate will cross cell membranes. • In general, the more highly charged a drug. The harder it is to cross a cell membrane. 11-1 Titration of strong base with strong acid • In the titration of any strong base with any strong acid, H+ + OH- → H2O there are three regions of the titration curve that require different kinds of calculations: 1. Before the equivalence point, the pH is determined by excess OH− in the solution. pO H log O H 2. At the equivalence point, H+ is just sufficient to react with all OH− to make H2O. The pH is determined by dissociation of water. O H H Kw 3. After the equivalence point, pH is determined by excess H+ in the solution. pH log H Example of titrating strong base with strong acid • Calculate the pH at each of the following points in the titration of 50.00 mL of 0.010 0 M NaOH with 0.100 M HCl. Volume of acid added: 0.00, 1.00, 2.00, 3.00, 4.00, 4.50, 4.90, 4.99, 5.00, 5.01, 5.10, 5.50, 6.00, 8.00, and 10.00 mL. • Answer: first, estimate the equivalence volume with the equation V(NaOH)*[NaOH] = V(HCl)*[HCl] ; V(HCl) = 5.00 ml 11.2 Titration of Weak Acid with Strong Base 1. 2. Before any base is added HA ↔ H + + A- Ka Before the equivalence point A pH pK a log HA 3. At the equivalence point, there is exactly enough NaOH to consume HA 4. After the equivalence point, pH is determined by the excess OH pO H log O H Titration curve of a weak acid by a strong base The following figures illustrate that as the acid becomes weaker or more dilute, the end point becomes less distinct. It is therefore not practical to titrate an acid or base when its strength is too weak or its concentration too dilute 11-3 Titration of a weak base with strong acids • Because the reactants are a weak base and a strong acid, the reaction goes essentially to completion after each addition of acid. 1. Before any acid is added, the solution contains only the weak base B + H2O ↔BH+ + OH- Kb 2. Between the initial point and the equivalence point: buffer! 3. At the equivalence point 4. Beyond the equivalence point: the pH is determined by the excess acid • The formal concentration of BH+, F′, is not the original formal concentration of B, because some dilution has occurred. • The solution contains BH+ at the equivalence point, so it is acidic. The pH at the equivalence point must be below 7. • Problem 11-14: A 100.0 mL aliquot of 0.100 M weak base B (pKb = 5.00) was titrated with 1.00 M HClO4. Find the pH at the following volumes of acid added: Va = 0, 1, 5, 9, 9.9, 10, 10.1, and 12 mL. • Solution: first calculate the equivalence volume Ve * 1.00 = 0.1 * 0.100 ; Ve = 0.01 L Compare Va with Ve and then select a corresponding equation from previous slide (details in class) 11-4 Titration in diprotic systems (a) Titration of 10.0 mL of 0.100 M base (pKb1 = 4.00, pKb2 = 9.00) with 0.100 M HCl. (b) Titration of 10.0 mL of 0.100 M nicotine (pKb1 = 6.15, pKb2 = 10.85) with 0.100 M HCl. 1: The two equivalence points are C and E. There is no sharp break at the equivalence point, J, because (??) 2: Points B and D are the halfneutralization points, whose pH values equal pKa2 and pKa1, respectively. 11.5 Finding the end point with a pH electrode Autotitrator delivers titrant from the bottle at the back to the beaker of analyte on the stirring motor at the right. Electrodes in the beaker monitor pH or concentrations of specific ions Finding the end point with a Gran plot • A problem with using derivatives to determine the end point is that titration data are least accurate near the end point. • Gran plot uses data from before the end point to find the end point. • Consider the titration of a weak acid: HA ↔ H+ + A- • Gran plot: A graph such as the plot of Vb · 10−pH versus Vb is used to find the end point of a titration. • Vb is the volume of base (titrant) added to an acid being titrated. The slope of the linear portion of the graph is related to the dissociation constant of the acid. 11-6 Finding the end point with indicators • Indicator itself is an acid or base whose various protonated species have different colors. • The pH range over which indicator changes its color is called the transition range. • The difference between the observed end point (color change) and the true equivalence point is called the indicator error. • In general, one seeks an indicator whose transition range overlaps the steepest part of the titration curve as closely as possible. pH indicator Practical notes and the leveling effect • Primary standards: substance that can be obtained pure enough to make accurate solution. • Other solutions need to be standardized against a primary standard. • Strong basic solutions attack glass and are best stored in plastic containers. • Level effect: refers to a solvent’s ability to level the effect of a strong acid or base dissolved in it. The acidity of a solvent that protonates the weak base, B, is defined as the Hammett acidity (H0) function • For dilute aqueous solutions, H0 approaches pH. For concentrated acids, H0 is a measure of the acid strength. The weaker a base, B, the stronger the acidity of the solvent must be to protonate the base. 11-9 Calculating titration curves with spreadsheets