Acid-Base Titration Review: Equivalence Points & pKa
advertisement
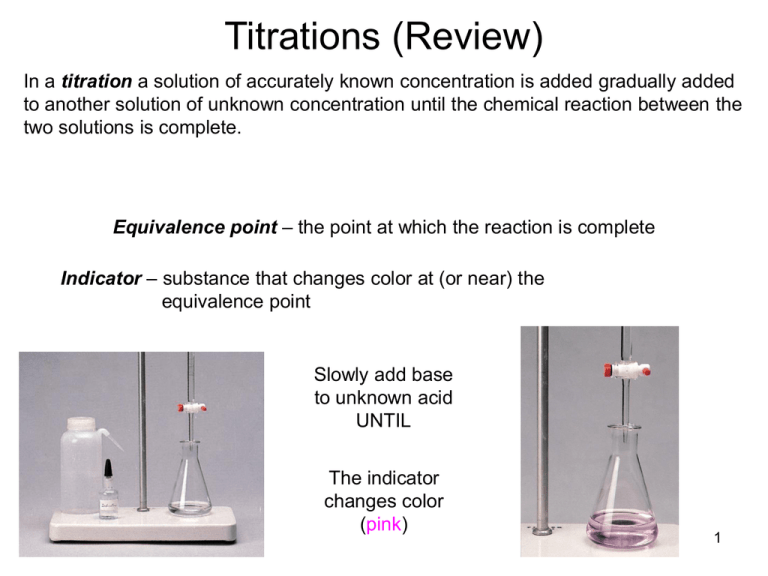
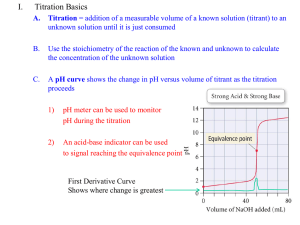
Titrations (Review) In a titration a solution of accurately known concentration is added gradually added to another solution of unknown concentration until the chemical reaction between the two solutions is complete. Equivalence point – the point at which the reaction is complete Indicator – substance that changes color at (or near) the equivalence point Slowly add base to unknown acid UNTIL The indicator changes color (pink) 1 Alternative Method of Equivalence Point Detection monitor pH 5 Strong Acid-Strong Base Titrations NaOH (aq) + HCl (aq) H2O (l) + NaCl (aq) OH- (aq) + H+ (aq) H2O (l) 7 Weak Acid-Strong Base Titrations CH3COOH (aq) + NaOH (aq) CH3COOH (aq) + OH- (aq) CH3COONa (aq) + H2O (l) CH3COO- (aq) + H2O (l) At equivalence point (pH > 7): CH3COO- (aq) + H2O (l) OH- (aq) + CH3COOH (aq) 8 Exp 16B – An Acid-Base Titration Curve Weak acid=strong base specific titration curves • • When titrating a weak acid with a strong base (or the other way around) the acid dissociation constant, Ka, for the weak acid can be determined from the titration curve It coincides with the middle of the “buffer region” of the titration curve Exp 16B – An Acid-Base Titration Curve Purpose of the experiment • • • • Perform an acid-base titration of potassium hydrogen phthalate (“KHP”) with sodium hydroxide, measure and record the changes in pH Plot the change of pH as a function of volume of base added Determine the Ka for the weak acid potassium hydrogen phthalate, KHP Determine the equivalence point Exp 16B – An Acid-Base Titration Curve Titration of potassium hydrogen phthalate with sodium hydroxide CO 2K CO 2K + + NaOH CO 2H H 2O CO 2Na potassium acid phthalate Standardization of an NaOH solution • NaOH needs to be standardized before it can be used in a titration – solid NaOH is hygroscopic and attracts H2O from the air • you cannot weigh NaOH accurately • you’ll also weigh absorbed water – an NaOH solution absorbs CO2 from the air, which will react to form carbonic acid, making the solution more acidic than the dissolved amount of NaOH suggests – standardization with potassium hydrogen phthalate (KHP) HC8H4O4-(aq) + OH-(l) C8H4O42-(aq) + H2O(l) – KHP is a weak acid, molar mass = 204.224 g/mol HC8H4O4-(aq) + H2O(l) C8H4O42-(aq) + H3O+(aq) Exp 16B – An Acid-Base Titration Curve Standardization of an NaOH solution • Equivalence point [H3O+] = [OH-] – If [H3O+] is known, [OH-] can be calculated Volacid x Molarityacid= Volbase x Molaritybase Vacid (L) x Macid (mol/L) = Vbase (L) x Mbase (mol/L) moles of acid = moles of base Prelab problem #2 25 mL 0.10 M NaOH neutralizes how many grams of KHP? KHP is a solid! Answer 25 mL 0.10 mol/L NaOH = 25 mL x 1L/1000 mL x 0.10 mol/L NaOH = ? mol NaOH At equivalence point: moles of KHP = moles of NaOH = ? mol ? mol KHP x 204.224 g/mol = ?? g KHP Exp 16B – An Acid-Base Titration Curve Determine pKa • Acid dissociation constant Ka = [H3O+] [A-]/[HA] [H3O+] = Ka [HA]/[A-] pH = -log [H3O+] = -log Ka + (-log [HA]/[A-]) = pKa - log [HA]/[A-] = pKa + log [base]/[acid] (Henderson-Hasselbalch equation) When is pH = pKa? • During a titration of the weak acid HA: HA(aq) + OH-(aq) A-(aq) + H2O(l) HA(aq)(acid) decreases because it reacts with OH-(aq), forming H2O A- (conjugate base) increases When [HA] = [A-], ½ of HA has reacted with OH pH = pKa + log [A-]/[HA] = pKa + log 1 = pKa This point is halfway between the start of the titration and the equivalence point Remember: at the equivalence point all acid has reacted with OH-: no HA is left Exp 16B – An Acid-Base Titration Curve Experimental 1. 2. 3. 4. Calibrate the pH meter. Use buffer pH 4 or pH 7 Clean the buret and rinse with water followed by a small amount of the NaOH Weigh between 0.50 – 0.53 g KHP. Record the exact mass to 4 decimal places! Dissolve KHP in 50.0 mL (exact!!) of dH2O in a 150 mL beaker*. Dissolve completely (why?) 5. Add 2 drops of phenolpthalein indicator (NOT done) 6. Immerse pH electrode in the KHP solution 7. Set up the buret and fill it with 50 mL 0.1 M NaOH solution. Be careful not to spill NaOH in the KHP solution! (Why does that matter? You are going to mix the solutions anyway)! Record the initial volume reading of the burette in your lab manual. The tip of the buret will be positioned slightly above the surface of the solution 8. Read and record the initial pH of the KHP solution before adding any NaOH 9. Start the titration by adding 1-mL aliquots of NaOH solution to the KHP solution while stirring. 10. Record the burette reading and the pH after each addition 11. When the pH changes fast (>0.3 units/mL of base) reduce the amount of base that you add to ~0.2 mL 12. You will quickly pass the equivalence point. When the pH changes start to lag again, return to the 1-mL additions. Keep titrating until the pH ~ 11.5-12 To get a good titration curve you need to go at least 5 mL beyond where the pH >11 13. Repeat the titration with a 2nd sample of KHP Acid-Base Titration Curve Exp 16B – An Acid-Base Titration Curve Calculations Sample Vol (equivalence point) (mL) pH (equivalence point) Vol at half-way point (mL) pH pKa Ka 1 2 Next Monday: Experiment 18 Spontaneity DUE Monday Nov. 19: Exp 16B: An Acid-Base Titration Curve • Datasheet, Calculations, Titration curves • Post lab questions 1-2 • Postlab question 3 (5 bonus points) Exp 18: Spontaneity • Prelab preparations – – – • Goal Procedure Physical and Chemical Properties of Ammonium nitrate Prelab Questions