Buffers and Titrations
advertisement

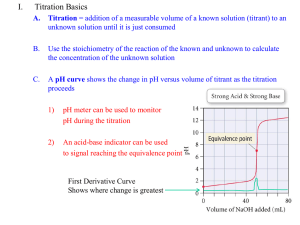
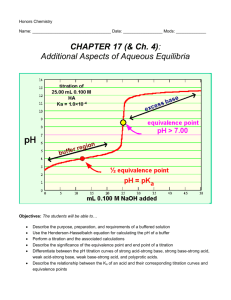
Buffers A Buffer in Action Definition of a Buffer A buffer solution is one which resists changes in pH when small quantities of an acid or a base are added to it. The most important way that the pH of the blood is kept relatively constant is by buffers dissolved in the blood. H2CO3 + H2O HCO3- + OHHCO3- + H+ H2CO3 HCO3- + 3OH H2O + H2CO3 When a substance acts as both an acid and a base such as HCO3- does, it is called amphoteric. How do buffer solutions work? A buffer solution contains a weak acid and base, which removes any hydrogen ions or hydroxide ions that are add – keeping the pH constant. (not neutral) The Bicarbonate Buffer: weak acid weak base H2CO3 + H2O HCO3- + OH- Another Buffer solution Ammonia is a weak base, and the position of this equilibrium will be well to the left: Any added hydrogen ions will react with the ammonia to make more of the conjugate acid, maintaining the pH. Another Buffer solutions Any added OH- ions will react with the weak acid to make the conjugate base and again maintain a constant pH. Determination of the pH of a Buffer solution Example: What is the pH of a solution of 0.11 M NaC2H3O2 and 0.090 M HC2H3O2? (ka = 1.8 x 10-5) HC2H3O2 C2H3O2- + H+ Initial 0.09 0.11 0 Change -x +X +x Equil. .09-x 0.11 +X x ka = x (0.11) /.09-x X = 1.47 x 10-5, pH = 4.83 (buffered solution will have a very small dissociation so + or – x can be ignored) Preparing Buffer Solutions The Henderson-Hasselbalch Equation gives the ratio of weak acid to base needed to maintain a constant pH. pH = pka + log [A-]/[HA] (The pH can be approximated: pH = pka +1 Preparing Buffers 1. Example. What is the mole ratio of acetic acid to acetate ion needed to prepare a buffer solution at a pH = 5.00? ka = 1.8 x 10-5 ka = [H+] [A-] = [HA] pH = 5 so, mol C2H3O2- = [H+] mol HC2H3O ka [H+] mol C2H3O2mol HC2H3O [H+] = 1 x 10-5 = 1x 10-5 1.8 x 10-5 = .56 Preparing Buffers 2. What is the pH of a solution containing 0.11 M of sodium acetate and 0.090 M acetic acid? Ka = 1.8 x 10-5 pH = pka + log [A-]/[HA] pH = 4.74 + log (0.11mol A-) (0.090 mol HA) pH = 4.83 Titrations Titration of a strong acid by a strong base The equivalence point occurs at 25.00 mL added base with a pH of 7.0. Titrations Titration of a weak acid by a strong base This can be divided into four regions 1) 2) 3) 4) Before the titration begins: this is simple a solution of weak acid During the titration, but before the equivalence point: the solution is a buffer At the equivalence point: the solution contains a salt of the weak acid, and hydrolysis can occur Past the equivalence point: the excess added OH- is used to determine the pH of the solution Buffered Region The titration curve for the titration of 25.00 mL of 0.200 M acetic acid with 0.200 M sodium hydroxide. Due to hydrolysis, the pH at the equivalence point higher than 7.00. Titrations Titration of a weak base by a strong acid This is similar to the titration of a weak acid by strong base Again dividing into four regions 1) 2) 3) 4) Before the titration begins: this is a solution of a weak base in water During the titration, but before the equivalence point: the solution is a buffer At the equivalence point: the solution contains the salt of the weak base, and hydrolysis can occur Past the equivalence point: excess added H+ determines the pH of the solution Buffered Region Titration curve for the titration of 25.00 mL of 0.200 M NH3 with 0.200 M HCl. The pH at the equivalence point is below 7.00 because of the hydrolysis of NH4+. Titrations Titration curves for diprotic acids The features are similar to those for monoprotic acids, but two equivalence points are reached The titration of the diprotic acid H2A by a strong base. As each equivalence point is reached, the pH rises sharply. Titrations A few general comments about indicators can be made Most dyes that are acid-base indicators are weak acids, which can be represented as HIn The color change can be represented as: HIn ( aq ) H ( aq ) In ( aq ) acid form base form (one color) (another K HIn color) [ H ][ In ] [ HIn ] Titrations The color change will “appear” to the human eye near the equivalence point of the indicator At the equivalence point, the concentration of the acid and base form are equal, so that pH at the equivalenc e point p K HIn The best indicators have intense color(s) so only a small amount will produce an intense color change that is “easy” to see and won’t consume too much of the titrant.