Titration Technique
advertisement

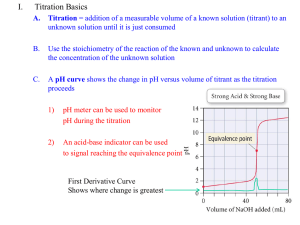
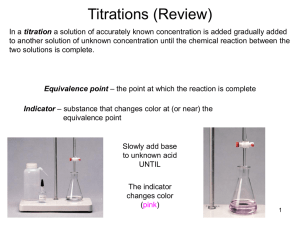
http://wps.prenhall.com/wps/media/o bjects/3312/3392202/blb1703.html Sites: http://chemed.chem.wisc.edu/chempaths/GenChemTextbook/Titrations-875.html IB: http://ibchem.com/IB/ibnotes/full/aab_htm/18.5.htm Simulation: http://www.avogadro.co.uk/chemeqm/acidbase/titration/phcurves.htm http://www.ausetute.com.au/titrcurv.html A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the "analyte") until the equivalence point is reached. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyte. An indicator may be added which has an "endpoint" (changes color) at the equivalence point, or the equivalence point may be determined from a titration curve. In an acid-base titration, a solution containing a known concentration of base is slowly added to an acid (or the acid is added to the base). Acid-base indicators can be used to signal the equivalence point of a titration (the point at which stoichiometrically equivalent quantities of acid and base have been brought together). The titrant is added to the solution from a buret, and the pH is continually monitored using a pH meter. To understand why titration curves have certain characteristic shapes, we will examine the curves for three kinds of titrations: (1) strong acid-strong base; (2) weak acid-strong base; a pH meter can be used to monitor the progress of the reaction producing a pH titration curve, a graph of the pH as a function of the volume of the added titrant. The shape of the titration curve makes it possible to determine the equivalence point in the titration. The titration curve can also be used to select suitable indicators and to determine the Ka of the weak acid or the Kb of the weak base being titrated. Titration – the progressive transfer of a solution from a buret (called the titrant) into a measured volume of another solution (called the sample). Equivalence point – the volume of titrant required to neutralize the sample (# mol acid = # mol base). Endpoint – the pH at the equivalence point of a titration, where the indicator changes color. Indicator – a chemical which is added to the sample that changes colour at the equivalence point of a titration. Buffering region – a horizontal region of the pH curve where pH is not changing significantly. The endpoint of a titration is NOT the same thing as the equivalence point: The equivalence point is a single point defined by the reaction stoichiometry as the point at which the base (or acid) added exactly neutralizes the acid (or base) being titrated. The endpoint is defined by the choice of indicator as the point at which the colour changes. Depending on how quickly the colour changes, the endpoint can occur almost instantaneously or be quite wide. If an appropriate indicator is chosen such that the endpoint of the titration occurs at the equivalence point, then a colour change in the solution being titrated can be used as a signal that the equivalence point has been reached. Intuition may suggest that the endpoint of the titration will occur at the equivalence point if we choose an indicator whose pKa is equal to the pH of the equivalence point. If such an indicator was chosen, the colour change would be half complete at the equivalence point. Thus for titrating a weak acid with a strong base where, at equivalence point the solution is slightly acidic, an optimum indicator should have a pKa > 7, for example Thymol Blue (pKa = 8.9) or Phenolphthalein (pKa = 9.4) For titrating a weak base with a strong acid at equivalence point encounters a slightly basic solution. Thus an optimum indicator should have a pKa < 7, for example Bromocresol Green (pKa = 4.7) or Methyl Red (pKa = 5.1) For titration involving strong base and a strong acid an optimum indicator should have a pKa ~ 7, for example Bromothymol Blue (pKa = 7.0) or Phenol Red (pKa = 7.9 1. Consider the titration when 0.100 M NaOH solution have been added to 50.00 mL of 0.100 M HCl. The initial pH—The pH of the solution before the addition of any base is determined by the initial concentration of the strong acid. For a solution of 0.100 M HCl [H+] = 0.100 M pH = -log(0.100) = 1.00 Between the initial pH and the equivalence point— ( adding 49 mL of NaOH) As NaOH is added, the pH increases slowly at first and then rapidly in the vicinity of the equivalence point. The pH of the solution before the equivalence point is determined by the concentration of acid that has not yet been neutralized. nH+ = 0.1 x 0.05 = 5 x 10-3 nOH- = 0.1 x 0.049 = 4.9 x 10-3 nH+ left = 5 x 10-3 – 4.9 x 10-3 = 1 x 10-4 mol H+ Vsolution = 50 + 49 = 99 mL = 0.099L [H+ ] = 1x10-4 / 0.099 = 0.001M => pH = 3 The equivalence point—At the equivalence point equal number of moles of the NaOH and HCl have reacted, leaving only a solution of their salt, NaCl. No calculation is required to deduce that the pH is 7.00, because the cation of a strong base (in this case Na+) and the anion of a strong acid (in this case Cl–) do not hydrolyze and therefore have no appreciable effect on pH. After the equivalence point—The pH of the solution after the equivalence point is determined by the concentration of the excess NaOH in the solution.(adding 51 mL of NaOH) V=50 + 51 = 101 mL = 0.101 L nH+ = 5 x 10-3 nOH- = 0.1 x 0.051 = 5.1 x 10-3 nOH- left = 5.1 x 10-3 – 5.0 x 10-3 = 1 x 10-4 mol OH[OH- ] = 1x10-4 / 0.101 = 9.9 x 10-4 M pOH = 3.00 pH = 11.00 3. What is the pH when 48.00 ml of .100 M NaOH solution have been added to 50.00 ml of .100 M HCl solution? As an example, consider the titration curve for the titration of 50.0 mL of 0.100 M acetic acid, HC2H3O2, with 0.100 M NaOH. The initial pH—This pH is just the pH of the 0.100 M HC2H3O2. The calculated pH of 0.100 M HC2H3O2 is 2.89 (using Ka) Between the initial pH and the equivalence point—To determine pH in this range, we must consider the neutralization of the acid Calculate the pH of the solution formed when 45.0 mL of 0.100 M NaOH is added to 50.0 mL of 0.100 M HC2H3O2 (Ka = 1.8 10–5). Consider the neutralization of the acid. Prior to reaching the equivalence point, part of the HC2H3O2 is neutralized to form C2H3O2–. Thus, the solution contains a mixture of HC2H3O2 and C2H3O2–. pH = 5.70 The equivalence point is reached after adding 50.0 mL of 0.100 M NaOH to the 50.0 mL of 0.100 M HC2H3O2. At this point the 5.00 10–3 mol of NaOH completely reacts with the 5.00 10–3 mol of HC2H3O2 to form 5.00 10–3 mol of their salt, NaC2H3O2. The Na+ ion of this salt has no significant effect on the pH. The C2H3O2– ion, however, is a weak base, and the pH at the equivalence point is therefore greater than 7. Finding the concentration of the salt : na = 0.005 nsalt = 0.005 = n C2H3O2V = 50 + 50 = 100 mL = 0.100 L Because the salt is a weak base Kb = Kw/Ka [OH- ] = 5.3 x 10-6 pH = 8.72