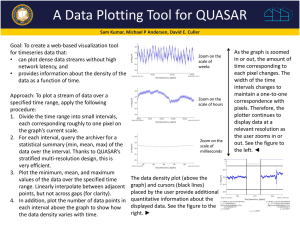
impedance spectroscopy: dielectric behaviour of
advertisement

IMPEDANCE SPECTROSCOPY: DIELECTRIC BEHAVIOUR OF POLYMER ELECTROLYTES By Ri Hanum Yahaya Subban Ph. D Faculty of Applied Sciences/Institute of Science UiTM Shah Alam OUTLINE • • • • • • • • • • • • IMPEDANCE SPECTROSCOPY (IS) BACKGROUND IS PRINCIPLE IS TECHNIQUE IS PLOT OF SIMPLE CIRCUITS IS PLOT OF MODEL SYSTEMS IS PLOT OF REAL SYSTEMS CONSTANT PHASE ELEMENT (CPE) IS PLOT OF REAL SYSTEMS AND CPE IMPEDANCE RELATED FUNCTIONS Z, Y AND M PLOTS FOR SIMPLE CIRCUITS SOME APPLICATION OF IS SOME PRACTICAL DETAILS FOR IS IS: BACKGROUND D. C METHOD A.C METHOD R=V/I cannot be used due to polarisation of charges - at electrode-electrolyte interface -at defect regions inside the sample (grain boundaries, phase boundaries etc. ) Polarisation effects are avoided and impedance (Z) is measured - Since Z changes with applied signal frequency, Z must be measured as a function of frequency and resistance of sample evaluated Also known as AC Impedance Spectroscopy Complex Impedance Spectroscopy Electrochemical Impedance Spectroscopy (when applied to electrochemical systems) Popular use of IS: To determine electrical conductivity of ionic conductors To identify different processes that contribute to the total conductivity: bulk contribution, grain boundary contribution, diffusion, etc. Through identifying an equivalent circuit for the impedance plot involved IS: BACKGROUND L • Resistance of sample A R=L A = resistivity of the material L = length of the sample A = area of cross-section of the sample • Conductivity = 1 = L/A R By measuring R,L and A, can be calculated IS: PRINCIPLE V(t) Sine wave signal V(t) = Vo sin t i(t) of low amplitude is applied to a sample Vo = maximum voltage = 2f, angular frequency The resulting current i(t) = io (sin t + ) = phase difference between i(t) and V(t) (current is ahead of voltage by ) : phase shift The impedance Z = V(t) = Vo sin (t) i(t) io (sin t + ) Z is a function of frequency and has magnitude Z = Vo = Zo and a phase angle io Both Z and are frequency dependent quantities PRINCIPLE: IS IS: PRINCIPLE • Since ac impedances are frequency dependent quantities they are represented by Z() Z() can be considered as a complex quantity with a real component Z’() and imaginary component Z”() Z() = Z’() + j Z”() , j =-1 where real impedance = Z’ = Z cos( ) imaginary impedance = Z” = Z sin( ) with a phase angle = tan-1 (Z”/ Z’) Complex Impedance plane Im. Z Z” Z Magnitude of Z, Z = [(Z’)2 + (Z”)2]1/2 Z’ Real Z IS: TECHNIQUE Small ac signal (V 10 mV) is applied to sample over a wide range of frequency (mHz to MHz) Liquid sample Solid sample Sample holder Electrode Sample Computer Impedance spectrometer: LCR meter/FRA Electrode IS: TECHNIQUE Measure Z(f) as a function of f(=2f) over a wide range of frequency (mHz to MHz) Plot Z(f) versus f in the form of -Z’’(f) vs Z’(f) for various f (Cole-Cole plot/ Complex impedance plot/Nyquist plot) Useful to evaluate : -electrical parameters such as conductivity of ionic conductors(solid or liquid), mixed conductors - electrode-electrolyte interfacial effects and related phenomena - electrochemical parameters/processes of the system under study Also used for studying dielectric behaviour of materials IS PLOT OF SIMPLE CIRCUITS a. Pure resistance R R Z = R for all values of or f Z = R and = 0 Z’ = R and Z” =0 Z” Z” R Impedance plot is a point on the real axis at Z’ = R Z’Z’ IS PLOT OF SIMPLE CIRCUITS b. Pure capacitance C C Z = 1 = -j jC C Z’ = 0 and Z” = -1 C Z = Z” varies with frequency As increases, Z decreases Z points lie along the Z” axis Z” -ve Z” Z’Z’ Impedance plot is a straight line lying on the Z” axis IS PLOT OF SIMPLE CIRCUITS c. R and C connected in series R C The total impedance Z=R- j C -ve Z” R Z’ With Z’ = R and Z” = -1 C On complex plane the graph becomes a straight line at Z’ = R, parallel to the Z” axis Z’ IS PLOT OF SIMPLE CIRCUITS d. R and C connected in parallel 1 = 1 + 1 = 1 + jC Z R 1/j C R Z= R R C 1 + j C = R(1 - jRC) = R(1 - jRC) 1 + (RC)2 (1 + jRC) (1 - jRC) = R - jR2C 1 + 2R2C2 = Z’ - 1 + 2R2C2 jZ” with Z” = RC Z’ On eliminating : (Z’- R/2)2 + (Z”)2 = (R/2)2 Equation of a circle IS PLOT OF SIMPLE CIRCUITS d. R and C connected in parallel -ve Z” Impedance plot is a semicircle with centre (R/2, 0) on the Z’ axis Maximum point on the semicircle corresponds to mRC = 1 m = 1 RC where RC = Time constant or Relaxation time m R/2 R Z’ Note: Z’ and Z” axes must have the same scales to see the semicircle From m , C can be calculated for an unknown circuit IS PLOT OF SIMPLE CIRCUITS e. Combined circuits Rs C1 C1 -ve Z” C2 -ve Z” Rs + R1 2 = R2C2 1 = R1C1 1 = R1C1 Rs R2 R1 R1 Z’ due to internal resistance of electrolyte/electrode interface R1 R1+ R2 Z’ IS PLOT OF MODEL SYSTEMS a. Ionic solid with two non-blocking electrodes Eg: Ag/AgI/Ag (Ag+ mobile, I- immobile) No ion accumulation at the electrodes Cell arrangement R and C connected in parallel (equivalent circuit) (assume no electrode resistance) Electrodes Sample Rb= bulk resistance Rb Cb Cb (Cg) bulk capacitance Expected impedance plot -ve Z” m Cb is related to vacuum capacitance Co; Cb = Co Z’ Rb/2 Rb And Co = oA d A - area of cross section - dielectric constant d - thickness of sample o – permittivity of free space IS PLOT OF MODEL SYSTEMS a. Ionic solid with two non-blocking electrodes: experimental results Rb Cb Cb(Cg) bulk capacitance (a) Li6SrLa2Ta2O12 with Li electrodes (b) Li6BaLa2Ta2O12 Thangadurai and Weppner Ionics 12 (2006) 81-92 Note: depressed/distorted semicircles IS PLOT OF MODEL SYSTEMS b. Ionic solid with two blocking electrodes Egs: AgI with Pt electrodes, Ag+ mobile and I- immobile Ions cannot enter the electrodes , get accumulated at the electrodes two double layer of charges at electrode/electrolyte interfaces two double layer capacitances at the interfaces ( C’dl) -ve Z” R m C’dl R/2 C R R Cdl + + + - Expected impedance plot Equivalent circuit C’dl + + + - - C Cdl= effective double layer capacitance Z’ Cdl will add a spike to the Impedance plot + IS PLOT OF MODEL SYSTEMS b. An ionic solid with two blocking electrodes: experimental results SS/PVC-LiCF3SO3/SS Au/Li6BaLa2Ta2O12/Au Thangaduarai and Weppner Ionics 12 ( 2006) 81-92 Subban and Arof, Journal of New Materials for Electrochemical Systems 6 (2003) 197-203 Note: depressed/distorted semicircles and slanted/curved spikes IS PLOT OF MODEL SYSTEMS c. Polycrystalline solid with two blocking electrodes Conduction will occur inside the grain (intra grain-bulk conduction) and along the grain boundaries (inter grain conduction) System = crystalline grain + grain boundaries + electrode/electrolyte interface -ve Z” Pt Grain boundary Z’ Rb Rgb Cb Equivalent circuit Cgb Pt Bulk Thickness of grain boundary is small large Cgb Rgb is large - larger semicircle for GB The overall σ : is determined by Rb +Rgb Rb+ Rgb Rb Electrode/electrolyte interface Grain Expected impedance plot Cdl IS PLOT OF MODEL SYSTEMS c. A polycrystalline solid with two blocking electrodes: experimental results 100 Hz 10 kHz R1 C1 From the values of the capacitances different semicircles can be associated with different conduction process in the sample R2 C2 High frequency semicircle (small C) bulk conduction Low frequency semicircle (large C) grain boundary conduction Cdl SS/ Li1+xCrxSn2-xP3-yVyO12/SS Norhaniza, Subban and Mohamed, Journal of Power Sources 244 (2013) 300-305 Note: Slanted/curved spike and depressed/distorted semicircles IS PLOT OF MODEL SYSTEMS TYPICAL C VALUES In general, a number of processes can contribute to the total conduction and an ideal equivalent circuit (hypothetical ) may be represented by the following simple circuit (various circuits possible) R1 C1 Bulk • • • R3 R2 C2 Grain boundary C3 Different phases or Orientation of crystal planes Cdl Double layer capacitance at the electrode R and C values ,particularly C values differ for different processes Each transport process may give a semicircle to the Impedance plot From the approximate C values different processes may be identified Approximate C values Phenomenon responsible 2-20 pF Bulk(main phase) 10 pF Second phase, orientation etc. 1-10 nF Grain boundary 0.1-10 Fcm-2 Double layer/surface charge 0.2 mFcm-2 Surface layer at electrode/adsorption Actual identification of different processes must be based on dependence on temperature, pressure, etc. IS PLOT OF REAL SYSTEMS IS plot of real systems and devices are usually complicated • Deviate from ideal behaviour due to : • Distorted semicircles may arise due to - Overlap of semicircles with various time constants • Depressed semicircles may arise due to - Electrolyte is not homogeneous - Distributed microscopic properties of the electrolyte • Slanted or curved spikes may arise due to - Unevenness of electrode/electrolyte interfaces - Charge transfer across the electrode/electrolyte interface, diffusion of species in the electrolyte or electrode The deviation from ideal behaviour of Impedance plot is explained in terms of a new circuit parameter called Constant Phase Element (CPE) CONSTANT PHASE ELEMENT (CPE) In general CPE has the properties of R and C (equivalent to a leaky capacitor) Mathematically impedance of a CPE is given by the Complex quantity: ZCPE = 1 = Zo (jω)-n , 0 ≤ n ≤ 1 Y0(jω) n When n = 0, Z is frequency independent and Zo R, CPE ≅ pure Resistance When n = 1, Z = 1 /jωY0 . Hence Yo C, CPE ≅ pure Capacitance CPE When 0 < n < 1, CPE acts as intermediate between R and C Can show that R and CPE in parallel gives a circular arc in the impedance plane as shown Usually CPE is denoted by the circuit element Q -Z” R -Q- • CPE alone gives an inclined straight line (pink) at angle (n=90) Q R n= 90° (1-n)= 90° C Z’ • CPE // R gives a tilted semicircle with its centre (C) depressed so that the plot appears as an arc (green) - The diameter of the semicircle is inclined at (1-n) = 90 IS PLOT OF REAL SYSTEMS AND CPE The general equivalent circuit of a solid electrolyte with non perfect blocking electrodes may take the form R1 R2 R3 CPE4 CPE1 -Z” CPE2 CPE3 Resulting impedance plot will have depressed semicircles and a slanted spike Here processes are assumed to be well separated Z’ R1 R1 + R2 R1 + R2 + R3 IMPEDANCE RELATED FUNCTIONS There are several other measured or derived quantities related to impedance (Z) which often play important role in IS: - Admittance (Y) - Dieletric/Permittivity (ε) - Modulus (electric) (M) Generally referred to as ‘immitances’ • The four different formalisms give the same information in different ways • However each formalism highlights different features of the system • Thus it may be worthwhile to plot the data in more than one formalism in order to extract all possible information from the results • Z plot gives prominence to most resistive elements • M plot gives prominence to smallest capacitance Eg: To study grain boundary effects , Z plot is good To study bulk effects M plot is good IMPEDANCE RELATED FUNCTIONS A.C voltage applied to a sample v = Zi Generally impedance Z = R + j X; R = resistance, X = reactance 1 Hence the current , i v Yv Z Immitance Symbol Relation Complex Form Impedance Z - Z’ – jZ” Admittance Y Y = Z-1 Y’ + j Y” Permittivity = 1/jCoZ = Y/ jCo ’ - j ” Electric modulus M M = -1 = jCo Z M’ + j M” Where Co oA d IMPEDANCE RELATED FUNCTIONS Complex Admittance Y ( ) 1 Z ( ) Y ' 1 Z ' ( ) jZ " ( ) Z' Z ' Z" 2 Z ' Z" 2 Z Y" 2 Z' '2 (Z 2 jZ " Z ' Z" 2 " Z "2 ) Complex Permittivity Z ' Co (Z " '2 Z "2 " ) Z Co (Z " M C o Z ' M M ' ( '2 Z "2 ) M ( ) Z ( ) C o Z j C o Z Complex Electrical Modulus '2 ' " " CoZ ) ' M " "2 " '2 " "2 ( ) ' 2 Z, Y AND M PLOTS FOR SIMPLE CIRCUITS C R M” -ve Z” Y” m Y’ 1/Rb C0/2C R Z’ M’ Z, Y AND M PLOTS FOR SIMPLE CIRCUITS R C Y” M” -ve Z” m R/2 M’ Co/2C Z’ R/2 m R 1/R Y’ u s v SOME APPLICATION OF IS DETERMINATION OF DC IONIC CONDUCTIVITY : - Z” vs Z’ OF IONIC CONDUCTORS 1.20E+03 PVC-NH4CF3SO3-Bu3MeNTf2N In general IS plot consists of a depressed semicircle with a tilted spike and intercept on the real axis corresponds to Rb 1.00E+03 Rb may be determined graphically by drawing the best semicircle OR by fitting R//C circuit with suitable values of R and C. Here the value R= Rb 8.00E+02 -Zi() Z i (Ω) R 6.00E+02 Note: both Z‘ and Z‘’ axes must have the same scale in order to see the semicircle . 4.00E+02 If only spike is present , it can be extended to obtain the intercept R 2.00E+02 p2 2 0.00E+00 0.00E+00 2.00E+02 4.00E+02 6.00E+02 8.00E+02 1.00E+03 1.20E+03 p1 2 Zr (Ω) S.K. Deraman Ph.D thesis UiTM 2014 is calculated from R by using =LA/Rb L - thickness of sample A - area of contact SOME APPLICATION OF IS DETERMINATION OF DC IONIC CONDUCTIVITY : - Z’’ vs. Z’ plot OF IONIC CONDUCTORS PVA-NH4x (x = Cl, Br, I) Equivalent circuit of PVA-NH4x at low NH4x concentration R1 CPE2 CPE1 A0 A5 Semi-circle disappears Only resistive component A5 prevails at higher frequency as NH4Br/I content increases Equivalent circuit of PVA-NH4x at high NH4x concentration A25 A30 Hema et. al J. Non Crystalline Solids 355 (2009) 84-90 CPE3 SOME APPLICATION OF IS ANALYSIS OF IS PLOT: CHOOSING EQUIVALENT CIRCUITS Choosing the correct equivalent circuit can be difficult Softwares do not give a unique equivalent circuit (model) for a particular IS plot but may suggest a number of complicated circuits (multiple models) Some possible equivalent circuits A An example: B Two time-constant impedance spectrum -Z” C Z’ B R1 R1 + R2 SOME APPLICATION OF IS ANALYSIS OF IS PLOT: CHOOSING EQUIVALENT CIRCUITS The model chosen should not only fit the IS data but also must be verifiable through other experiments, theories and justifiable through other known facts , etc. An example: The circuits below can give 3 distinct semi-circles in the IS plot if their time constants are well separated b a C1 R1 Cg C2 R2 C3 R3 Can be equivalent to CR Rg C2 RR R2 a is more suitable for a polycrystalline sample b is more suitable for a homogeneous material SOME APPLICATION OF IS DETERMINATION OF DIELECTRIC : ’ vs log f (permittivity/ PARAMETERS OF IONIC CONDUCTORS dielectric constant, transport processes ) Low frequency: Static dielectric constant 10 4 High frequency: Optical dielectric constant at 104Hz between 3.5 and 5 Compared to pure PVC film = 3 PVC(1-x)LiCF3SO3xLiPF6 Subban and Arof Ionics 9 (2003) 375-381 ε’ : • a measure of a material’s polarisation • associated with capacity to store charge and •represents the amount of dipole alignment in a given volume • related to dielectric relaxation In ionic conductors: Relaxation peaks usually not observed due to large electrode polarisation effects Alternative is M’ /M” or ac conductivity SOME APPLICATION OF IS DETERMINATION OF DIELECTRIC PARAMETERS OF IONIC CONDUCTORS : ’ vs concentration Same trend: variation with concentration PCL-NH4SCN Woo et. al Materials Chemistry and Physics 134 (2012) 755-761 SOME APPLICATION OF IS DETERMINATION OF DIELECTRIC : M” vs log f (relaxation PARAMETERS OF IONIC CONDUCTORS time , transport processes ) PEO- AgCF3SO3 Relaxation peaks Relaxation peak is responsible for fast segmental motion which reduces the relaxation time and increase the transport properties fmax Gondaliya et.al Materials Sciences and Applications 2 (2011) 16391643 Relaxation time =1/2fmax SOME APPLICATION OF IS DETERMINATION OF DIELECTRIC PARAMETERS OF IONIC CONDUCTORS : Tan = ”/‘ Tan vs log f (relaxation time and nature of conductivity relaxation) •Single well defined resonance peak is an indication of long range conductivity relaxation in good ionic conductors •From FWHM value can find out Debye conformation (=1.14) or otherwise. ( t ) exp[ ( t M ) ], 1 . 14 FWHM Effect of temperature Effect of concentration MG30-LiCF3SO3 Yap et. al, Physica B 407(20120 2421-2428 SOME APPLICATION OF IS Number density of charge DETERMINATION OF IONIC carriers h, mobility , TRANSPORT PARAMETERS IN IONIC : diffusion coefficient D, CONDUCTORS transference number t ion etc, s = hem Chitosan-LiClO4-TiO2-DMC æ 2(Ze2 ) ö s =ç ÷h Eat exp(-Ea / kT ) è 3kTm ø Ea kT o exp gradient Ea k Muhammad et. al, Key Engineerinhg Materials 594-595 (2014) 608-612 SOME APPLICATION OF IS Number density of charge DETERMINATION OF IONIC carriers h, mobility , TRANSPORT PARAMETERS IN IONIC : diffusion coefficient D, CONDUCTORS transference number t ion etc, 1/ 2 l v 2Ea v m Eg. For CMC l 1 . 5 nm CMC-NH4Br l v kT D 2 he H+ CMC Sample with optimised conductivity Samsudin and Isa, J. of Applied Sciences 12 (2012) 174-179 SOME APPLICATION OF IS DETERMINATION OF IONIC CONDUCTION MODEL : log () - dc vs. log () (exponent s) ac o r ac A dc s ac Gradient = s Small Polaron Hopping (SPH ) model Samsudin and Isa, J. of Current Engineering Research 1(2) (2011) 7-11 SOME PRACTICAL DETAILS FOR IS Frequency window limitation: the available equipment have limited frequency range: fl to fh •Only part of IS spectrum is obtained (depends on R and C value) •Changing the temperature may show different parts of the full spectra provided no new conduction processes comes into play at different temperatures •Curve fitting is needed to see full spectrum