Chem 5336_Potentiometry
advertisement

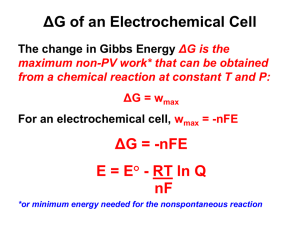
Summary of Potentiometry: pH and Ion Selective Electrodes Potentiometric Sensors I = controlled at 0 Amps Eeq is measured In general Eeq = const – 0.0592 log aX Electrode Potentials (review) • Electrochemical cell – two half cells • Convention, write both as reductions • 2 AgCl (s) + 2 e- = 2 Ag (s) + 2 Cl- (cathode) • - [2 H+ + 2 e- = H2 (gas)] (Pt, NHE reference, anode) Cell reaction is the sum of the two above 2 AgCl (s) + H2 = 2 Ag (s) + 2 Cl- + 2 H+ • Ecell = Ecathode - Eanode Ecell = EAg/AgCl – EH+/H2 by convention EH+/H2 = 0 Ecell = EAg/AgCl = 0.46 V DG = - n F Ecell ; DG = positive, non-spontaneous, electrolytic cell Measurements in Potentiometry; I = 0 Amps; equilibrium Cell: working electrode + reference electrode (E half cell = const) Working or indicator Ecell = EWE – Eref - Ejunction Simple potentiometric measuring circuit Variable resistor V voltmeter galvanometer Move slidewire (arrow) until G shows I = 0, then V = Ecell = Eeq In practice this is all automatic in modern potentiometers or pH meters Reference electrodes: critical to both potentiometry and voltammetry They keep a nearly constant half cell potential during experiment Normal Hydrogen, Pt| H2 (1 atm), HCL (0.01 M), NHE THE STANDARD, E = 0 V, but not practical Standard Calomel Electrode Hg| Hg2Cl2 (s), KCl (sat’d.) SCE Set up as self-contained Half cell Contact to test solution Half cell potential of the SCE – serves as a reference against which other E’s are measured Hg2Cl2 (s) + 2 e- = 2 Hg (l) + 2 ClUse Nernst equation: E = Eo - [RT/nF] ln (aCl2 aHg2/acalomel) ; but a of pure solids =1 only aCl remains in the log term, and E = Eo’ - [0.0592/2] log [Cl-]2 or E = Eo’ - 0.0592 log [Cl-] ; sat’d KCl is ~3.5 M at 25 oC So ESCE = 0.244 V vs. NHE at 25 oC Alternative reference: Ag|AgCl (s), KCl (sat’d.) EAg/AgCl = 0.199 V vs. NHE at 25 oC Half cell potential of the SCE – serves as a reference against which other E’s are measured Hg2Cl2 (s) + 2 e- = 2 Hg (l) + 2 ClUse Nernst equation: E = Eo - [RT/nF] ln (aCl2 aHg2/acalomel) ; but a of pure solids =1 only aCl remains in the log term, and E = Eo’ - [0.0592/2] log [Cl-]2 or E = Eo’ - 0.0592 log [Cl-] ; sat’d KCl is ~3.5 M at 25 oC So ESCE = 0.2415 V vs. NHE Alternative reference: Ag|AgCl (s), KCl (sat’d.) EAg/AgCl = 0.2415 V vs. NHE Ion Selective Electrodes (ISE) - sensor surface usually a membrane That adsorbs the ions, Eeq measured at I = 0 Amps In pH electrode, the membrane is a very thin glass layer 0.1 M HCl Stand alone glass pH electrode must be used with reference Glass pH electrode combined with internal reference electrode Glass membranes are made of SiO2, Li2O (or Na2O) and BaO (or CaO) ISE’s obey Nernst-like equations (25 oC) E = const + [0.0592/z] log aion if the ion Is H+, E = const - 0.0592 pH; ISE measure activity, not conc. pH = -log aH+ Fast response is important, < 1 s in buffer for most pH electrodes ISE Nernstian region, slope = 0.0591/z E, mV Log aion Most pH meters read pH directly, But must be calibrated daily How a glass pH electrode responds to H+ ions (must store in in water or buffer to maintain hydrated layer) Ag/AgCl 0.1 N HCl aH+ = const Inner hydrated layer a2 0.1 mm E2 Dry glass outer hydrated layer Test solution aH+ , soln a1 50 mm E1 EM = E1 – E2 + Eint ref; or EB = E1 – E2 (boundary E) Ecell = const + EB Li+ in glass can exchange with H+ (both small ions), giving rise to E1 and E2 H+ DOES NOT cross the membrane EB is related to a1 and a2, but a2 is constant (0.1 M HCl) These ion activities control the membrane potentials, E1 and E2 and so control EB, at 25 oC EB = E1 – E2 = 0.0592 log (a1/a2) Ecell = const + 0.0592 log (a1) Ecell = const - 0.0592 pH In practice, pH meter incorporates these equations And relates them to measurements with standard buffer, And the output is a direct measurement of pH: pH = pHstd + F (Ecell - Estd)/2.303 RT, R = gas const, T = abs. temperature Errors and Interferences in pH electrode measurements Alkaline error: In basic solutions or high salt conc. NaCl, KCl, Na+ and K+ interfere by adsorbing to the glass membrane then pHobs < true pH e.g. 0.1 M NaOH, pH 13, [Na+] is 0.1 M, [H+]= 1 x 10-13 Large error due to high [Na+], low [H+] In general fpr ISEs, Nicolsky equation Ecell = const + 0.0592 log [a1 + Σ Kjaj], j = 1….n interfering ions Kj = selectivity coefficient Acid error pH < 1, origin unknown pH electrodes reliable between pH 1 and 13 only ISE’s for ions other than H+ • glass membranes, Na+, K+, NH4+ - different composition than pH • solid state membranes, F-, S• liquid membranes, Ca+ • gas sensitive electrodes CO2, H2S, NH3 • enzyme electrodes – biological molecules • can all be used with pH meter in mV-mode Fluoride ISE – Solid State FLUORIDE ISE Detection limit 10-9 M Impregnated with ion exchanger; Ca++ ISE, Ca(dodecylphosphate) + Polymer like PVC; Phosphate groups bind Ca++ Detection limit Crystals or solid state have the analyte Ion present, e.g. LaF3 ~10-8 to 10-6 M Urea Enzyme ISE (NH)2CO + 2 H2O + H+ Urease enzyme 2 NH4+ + HCO3- Detection limit ~10-6 M